 |
HMT710S - Hydrometallurgy 314 - 2nd Opp - June 2022 |
 |
1 Page 1 |
▲back to top |

n Am I BI A u n IVER s I TY
OF SCIEnCE Ano TECHnOLOGY
FACULTY OF ENGINEERING AND SPATIAL SCIENCES
DEPARTMENT OF MINING AND PROCESSENGNEERING
QUALIFICATION : BACHELOR OF ENGINEERING IN METALLURGY
QUALIFICATION CODE: 08BEMT
LEVEL: 7
COURSE CODE: HMT 710S
COURSE NAME: HYDROMETALLURGY 314
SESSION: JUNE 2022
DURATION: 2 HOURS
PAPER: THEORY
MARKS: 60
SECOND OPPORTUNITY QUESTION PAPER
EXAMINER{S) Mr. Bernard Sililo
MODERATOR: Dr. Theresa Coetsee
INSTRUCTIONS
1. Answer all questions.
2. Read all the questions carefully before answering.
3. Marks for each question are indicated at the end of each question.
4. Please ensure that your writing is legible, neat and presentable.
PERMISSIBLE MATERIALS
1. Examination paper.
THIS QUESTION PAPER CONSISTS OF 4 PAGES (Including this front page)
 |
2 Page 2 |
▲back to top |

 |
3 Page 3 |
▲back to top |
Question 1
[12]
1.1 Differentiate between electrowinning and electrorefining.
(4)
1.2 The efficiency of the hydrogen reduction reaction can have a major influence on the
viability and efficiency of electrowinning.
a) Use zinc electrowinning to explain that statement.
(4)
b) Discuss how the substrate and other process conditions like pH may influence the
hydrogen reduction reaction.
(4)
Question 2
[12]
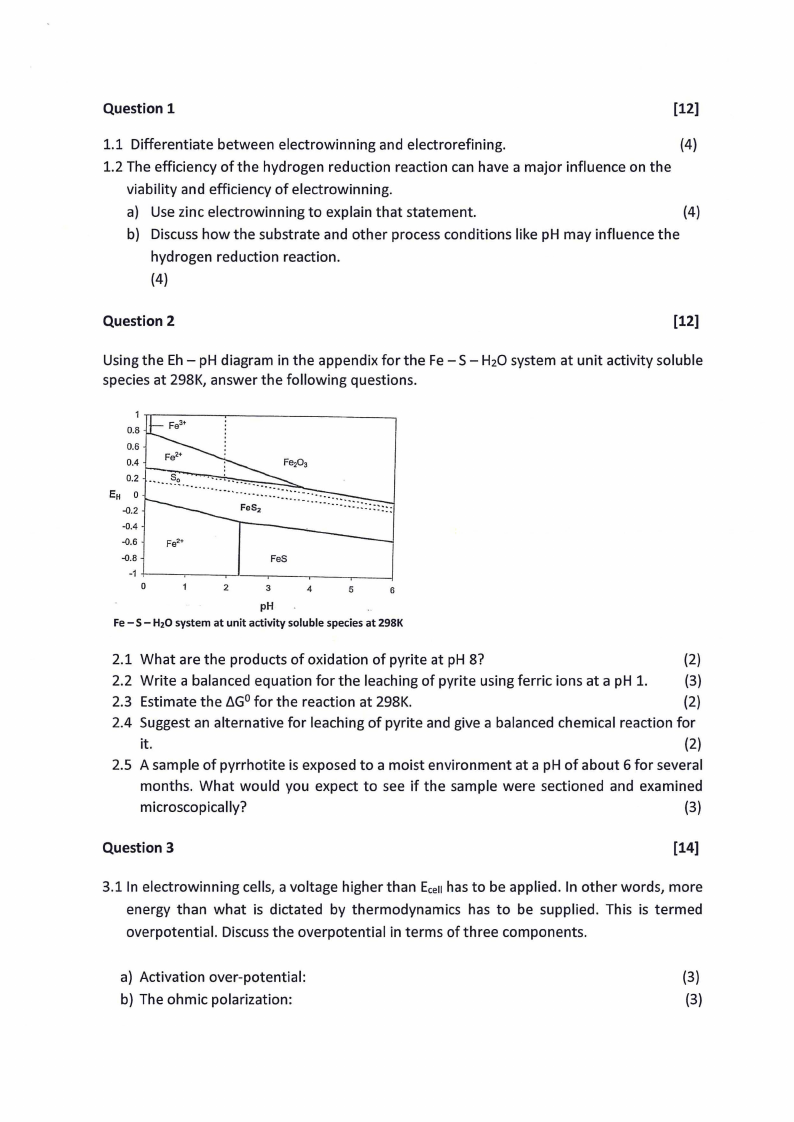
Using the Eh- pH diagram in the appendix for the Fe- S- H20 system at unit activity soluble
species at 298K, answer the following questions.
0.8
0.6
0.4
0.2
EH 0
-0.2
-0.4
-0.6
Fe 2+
-0.8
FeS
-1 +-, ----,~--,--l'--.-------.--.-----l
0
2
3
4
5
6
pH
Fe- S- H20 system at unit activity solublespeciesat 298K
2.1 What are the products of oxidation of pyrite at pH 8?
(2)
2.2 Write a balanced equation for the leaching of pyrite using ferric ions at a pH 1.
(3)
2.3 Estimate the D.G0 for the reaction at 298K.
(2)
2.4 Suggest an alternative for leaching of pyrite and give a balanced chemical reaction for
it.
(2)
2.5 A sample of pyrrhotite is exposed to a moist environment at a pH of about 6 for several
months. What would you expect to see if the sample were sectioned and examined
microscopically?
(3)
Question 3
[14]
3.1 In electrowinning cells, a voltage higher than Ecelhl as to be applied. In other words, more
energy than what is dictated by thermodynamics has to be supplied. This is termed
overpotential. Discussthe overpotential in terms of three components.
a) Activation over-potential:
(3)
b) The ohmic polarization:
(3)
 |
4 Page 4 |
▲back to top |

 |
5 Page 5 |
▲back to top |
c) Concentration overpotential:
(3)
3.2 Discuss how and when mass transfer of metal ions to an electrode may become the rate
limiting process in the electrowinning of that metal at the electrode. Also indicate how
you would define the maximum rate of metal deposition in terms of the relevant
parameters.
(5)
Question 4
[7]
Blister copper that is produced is normally treated using the copper electrorefining process.
4.1 Explain why this is the case.
(2)
4.2 Discuss the factors that determine the operating current density in copper
electrorefining.
(5)
Question 5
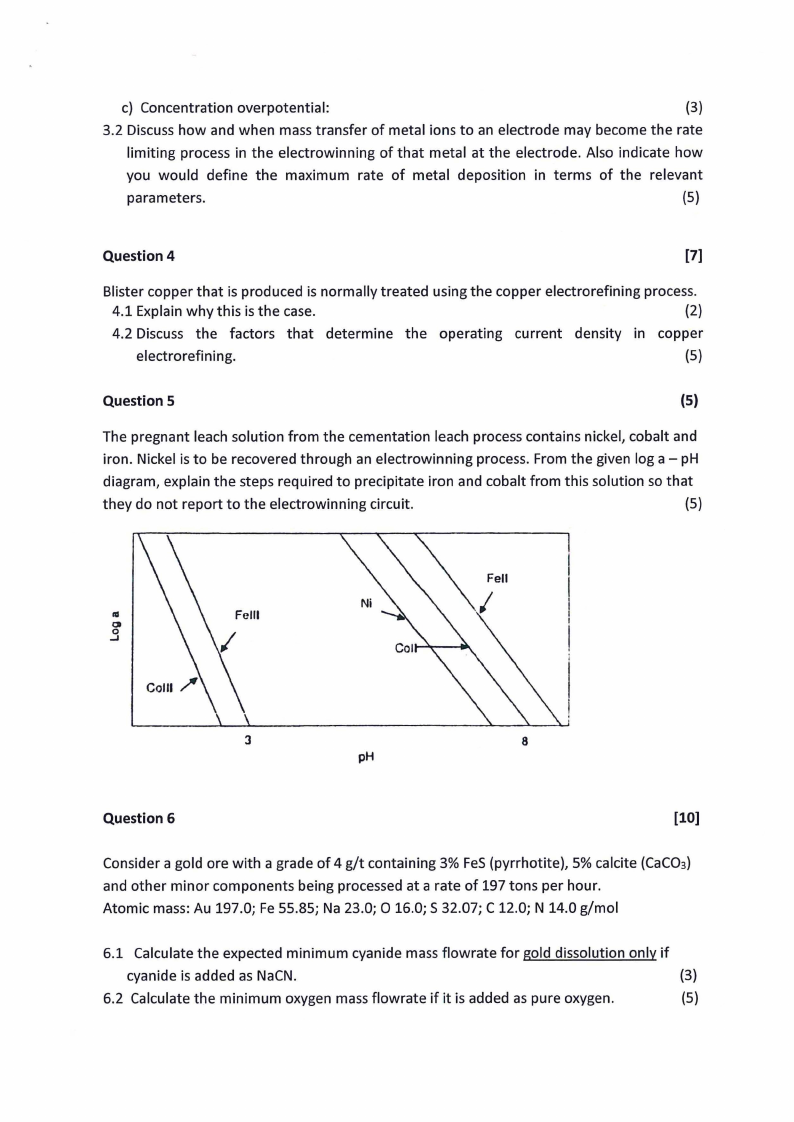
(5)
The pregnant leach solution from the cementation leach process contains nickel, cobalt and
iron. Nickel is to be recovered through an electrowinning process. From the given log a - pH
diagram, explain the steps required to precipitate iron and cobalt from this solution so that
they do not report to the electrowinning circuit.
(5)
Ill
OIi
0
...J
3
8
pH
Question 6
[10]
Consider a gold ore with a grade of 4 g/t containing 3% FeS(pyrrhotite), 5% calcite (CaC03)
and other minor components being processed at a rate of 197 tons per hour.
Atomic mass: Au 197.0; Fe 55.85; Na 23.0; 0 16.0; S 32.07; C 12.0; N 14.0 g/mol
6.1 Calculate the expected minimum cyanide mass flowrate for gold dissolution only if
cyanide is added as NaCN.
(3)
6.2 Calculate the minimum oxygen mass flowrate if it is added as pure oxygen.
(5)
 |
6 Page 6 |
▲back to top |

 |
7 Page 7 |
▲back to top |

6.3 Would you expect the actual required flowrates to be what you calculated? Defend your
answer.
(2)
 |
8 Page 8 |
▲back to top |






