 |
MSC701S- MOLECULAR SPECTROSCOPY AND CHEMICAL SEPERATION METHODS - 1st OPP - JUNE 2022 |
 |
1 Page 1 |
▲back to top |

NAMIBIA UNIVERSITY
OF SCIENCE AND TECHNOLOGY
FACULTY OF HEALTH, APPLIED SCIENCES AND NATURAL RESOURCES
DEPARTMENT OF NATURAL AND APPLIED SCIENCES
QUALIFICATION: BACHELOR OF SCIENCE
QUALIFICATION CODE: 07BOSC
~ | LEVEL: 7
COURSE CODE: MSC701S
COURSE NAME: MOLECULAR SPECTROSCOPY AND
CHEMICAL SEPARATION METHODS
SESSION: JUNE 2022
DURATION: 3 HOURS
PAPER: THEORY
MARKS: 100
FIRST OPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S) | DR JULIEN LUSILAO
MOpERATOR: | DR STEFAN LOUW
INSTRUCTIONS
1. Answer ALL the questions in the answer book provided.
2. Write and number your answers clearly.
3. All written work MUST be done in blue or black ink.
PERMISSIBLE MATERIALS
Non-programmable calculators
ATTACHMENTS
List of Useful formulas and Constants
THIS QUESTION PAPER CONSISTS OF 8 PAGES (Including this front page and attachments)
Page 1 of 8
 |
2 Page 2 |
▲back to top |
Question 1
[25]
1.1 Define the following terms
(a) Detectors
(2)
(b) Transducers
(2)
(c) Radiant power (or intensity)
(2)
1.2 It is known that transmittance (T) and absorbance (A) cannot normally be measured
accurately with spectrometric instruments. Provide reasons behind this limitation. (3)
1.3 What is the experimental approach used by analytical chemists to circumvent the
limitation mentioned in 1.2?
(4)
1.3 What is the difference in the bandwidth obtained in atomic and molecular
spectroscopy? Give the reason behind that difference.
(3)
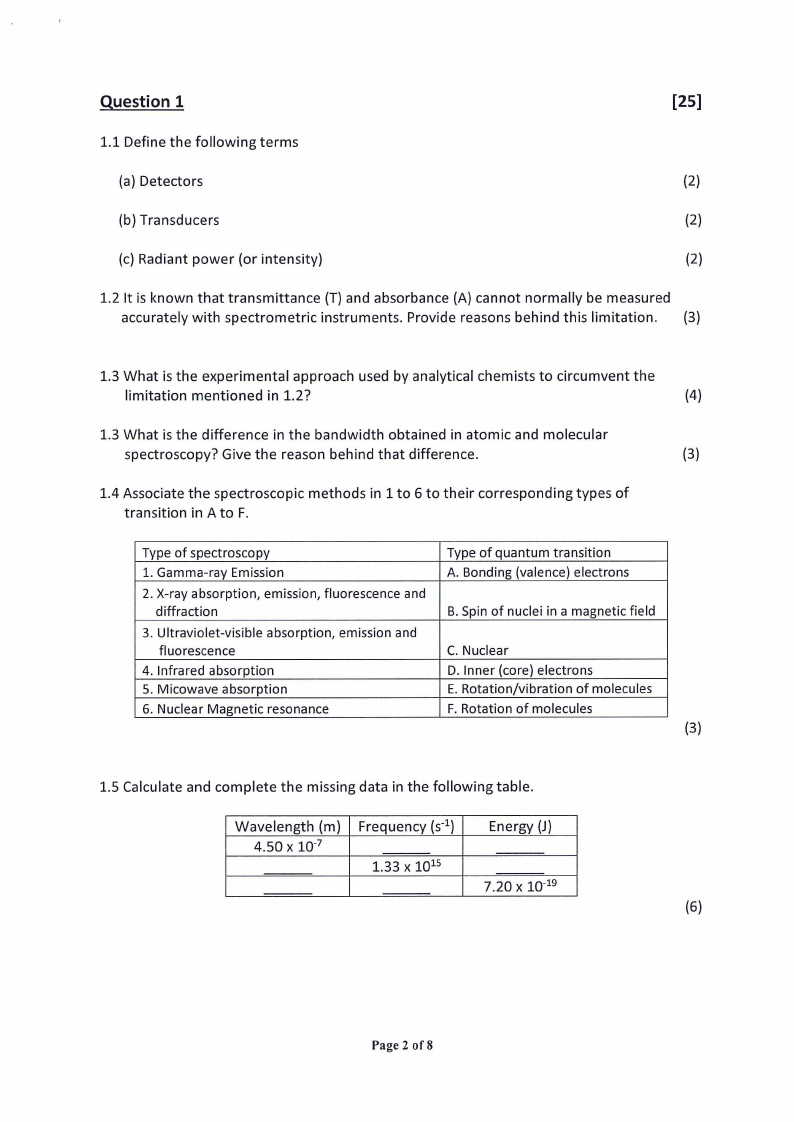
1.4 Associate the spectroscopic methods in 1 to 6 to their corresponding types of
transition in A to F.
Type of spectroscopy
1. Gamma-ray Emission
Type of quantum transition
A. Bonding (valence) electrons
2. X-ray absorption, emission, fluorescence and
diffraction
B. Spin of nuclei in a magnetic field
3. Ultraviolet-visible absorption, emission and
fluorescence
C. Nuclear
4. Infrared absorption
D. Inner (core) electrons
5. Micowave absorption
E. Rotation/vibration of molecules
6. Nuclear Magnetic resonance
F. Rotation of molecules
(3)
1.5 Calculate and complete the missing data in the following table.
Wavelength (m) | Frequency (s**)
4.50 x 10°”
1.33 x 10%
Energy (J)
7.20 x 10°19
Page 2 of 8
 |
3 Page 3 |
▲back to top |
Question 2
[25]
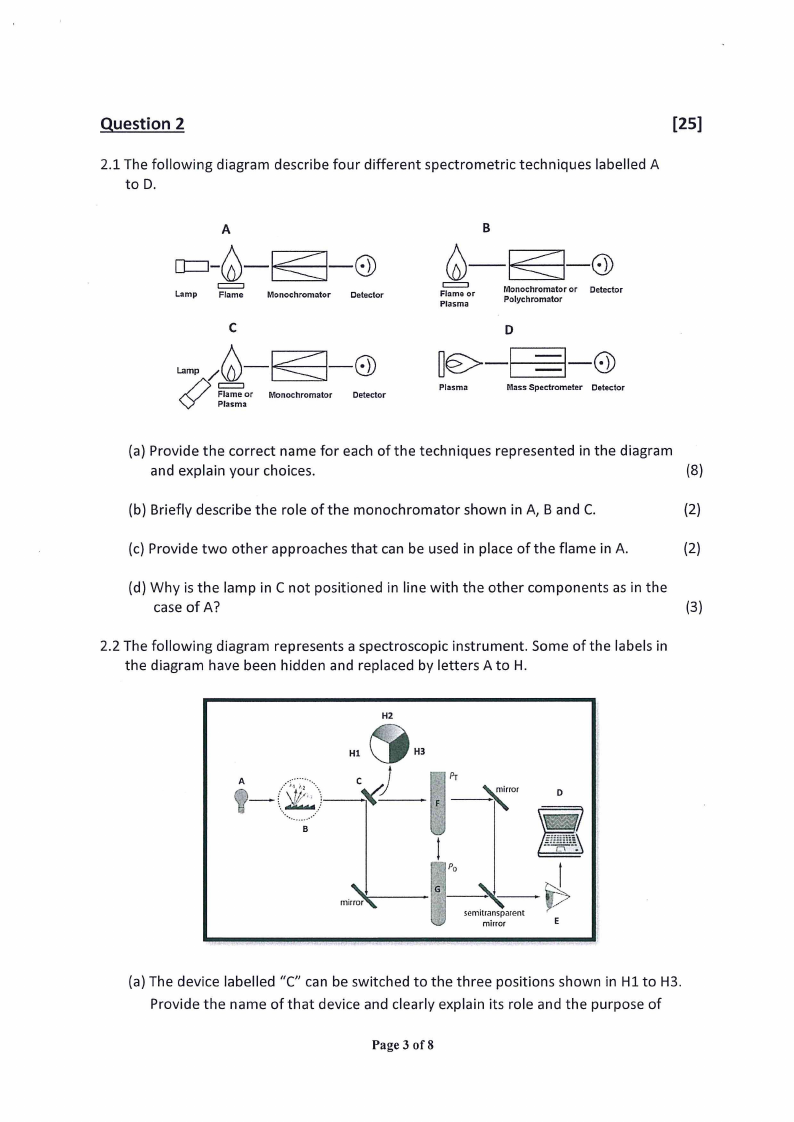
2.1 The following diagram describe four different spectrometric techniques labelled A
to D.
A
B
—
Lamp
Flame
Monochromator Detector
c
=)
FClc ame or
Plasma
Monochromator
oe
Detector
co
Flame or
Plasma
won
ee or Detector
v
D
e—-o
Plasma
Mass Spectrometer
Detector
(a) Provide the correct name for each of the techniques represented in the diagram
and explain your choices.
(8)
(b) Briefly describe the role of the monochromator shown in A, B and C.
(2)
(c) Provide two other approaches that can be used in place of the flame in A.
(2)
(d) Why is the lamp in C not positioned in line with the other components as in the
case of A?
(3)
2.2 The following diagram represents a spectroscopic instrument. Some of the labels in
the diagram have been hidden and replaced by letters A to H.
\\ mirror
D
mirror
semitransparent
mirror
|> >
(a) The device labelled “C” can be switched to the three positions shown in H1 to H3.
Provide the name of that device and clearly explain its role and the purpose of
Page 3 of 8
 |
4 Page 4 |
▲back to top |
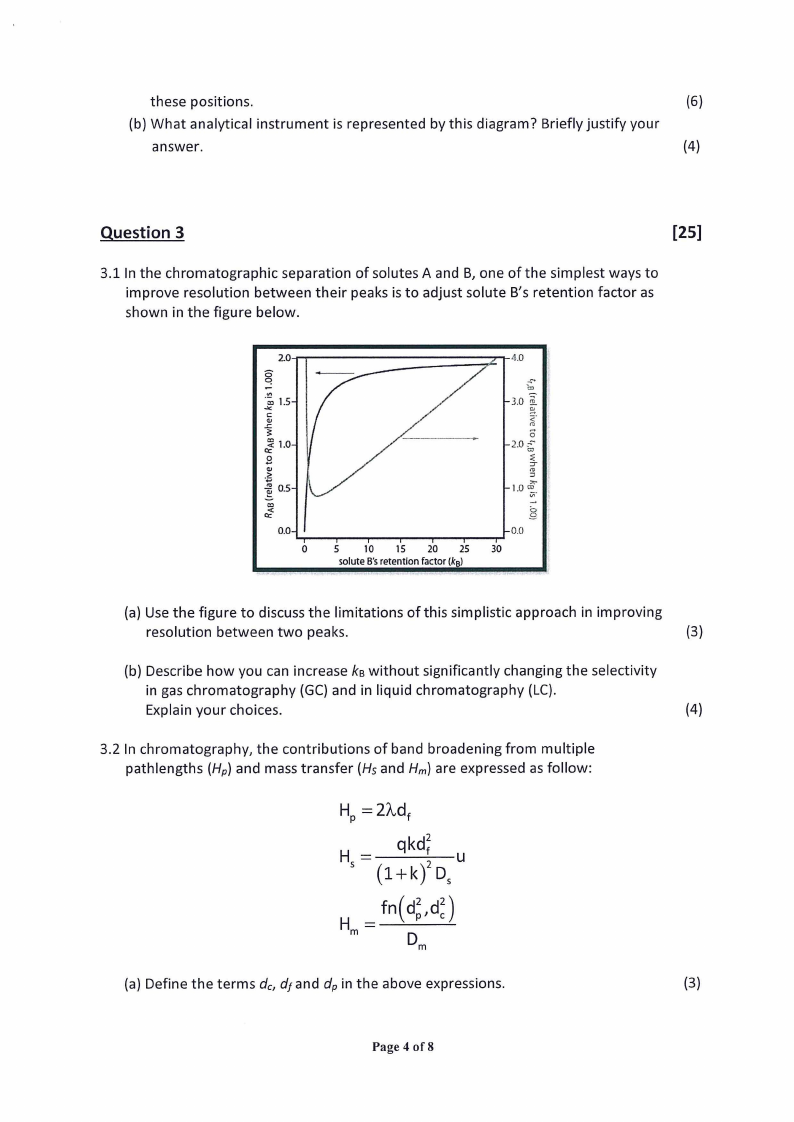
these positions.
(6)
(b) What analytical instrument is represented by this diagram? Briefly justify your
answer.
(4)
Question 3
[25]
3.1 In the chromatographic separation of solutes A and B, one of the simplest ways to
improve resolution between their peaks is to adjust solute B’s retention factor as
shown in the figure below.
S2=
‘2
xZc
ESose
°
&2-o
©22
0.0~
qT
0
q
T
U
¥
T
5
10
18
20
25
solute B’s retention factor (kg)
m=
o
6o=a.
B=3
=
a=es)=<
8=wn
- 0.0
tT
30
(a) Use the figure to discuss the limitations of this simplistic approach in improving
resolution between two peaks.
(3)
(b) Describe how you can increase kg without significantly changing the selectivity
in gas chromatography (GC) and in liquid chromatography (LC).
Explain your choices.
(4)
3.2 In chromatography, the contributions of band broadening from multiple
pathlengths (Hp) and mass transfer (Hs and Hm) are expressed as follow:
H, =2Ad,
H, = _ kd?i—u
(1+k) D,
4 fn( d22 ck 42)
m
D..
(a) Define the terms dc, dg and dp in the above expressions.
(3)
Page 4 of 8
 |
5 Page 5 |
▲back to top |
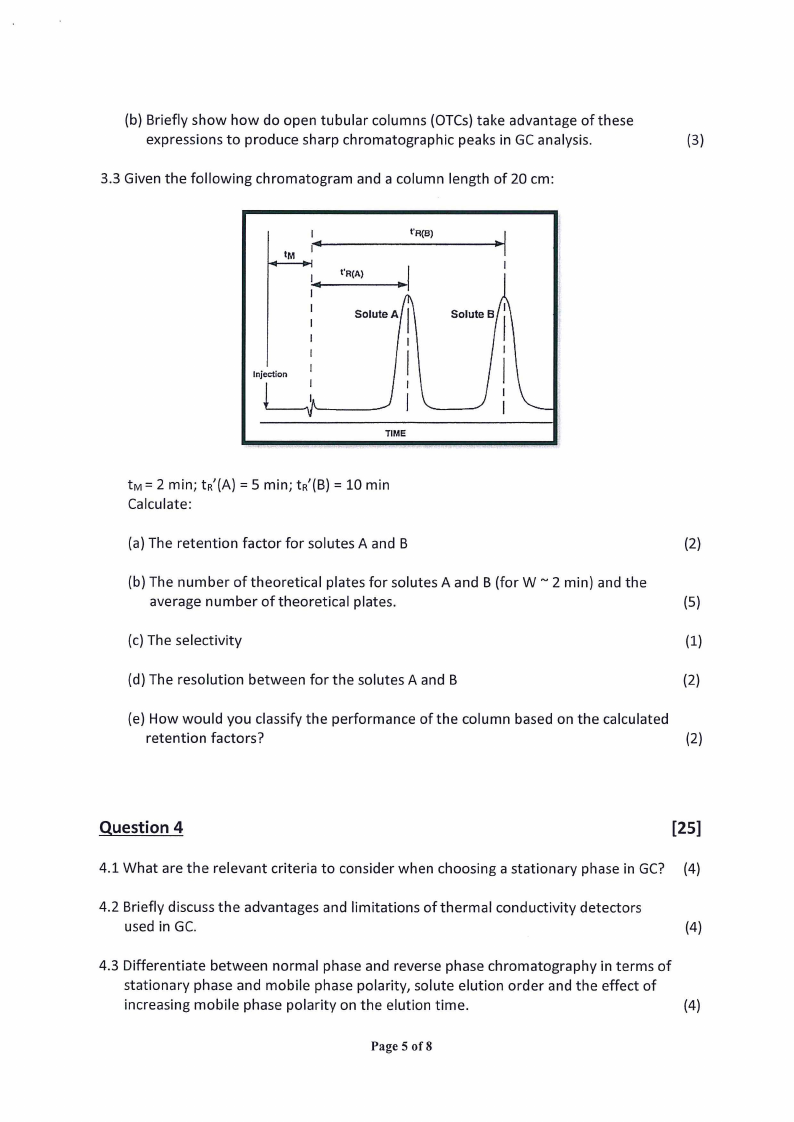
(b) Briefly show how do open tubular columns (OTCs) take advantage of these
expressions to produce sharp chromatographic peaks in GC analysis.
(3)
3.3 Given the following chromatogram and a column length of 20 cm:
Injection
tw= 2 min; tr’(A) = 5 min; tr’(B) = 10 min
Calculate:
(a) The retention factor for solutes A and B
(2)
(b) The number of theoretical plates for solutes A and B (for W ~ 2 min) and the
average number of theoretical plates.
(5)
(c) The selectivity
(1)
(d) The resolution between for the solutes A and B
(2)
(e) How would you classify the performance of the column based on the calculated
retention factors?
(2)
Question 4
[25]
4.1 What are the relevant criteria to consider when choosing a stationary phase in GC? (4)
4.2 Briefly discuss the advantages and limitations of thermal conductivity detectors
used in GC.
(4)
4.3 Differentiate between normal phase and reverse phase chromatography in terms of
stationary phase and mobile phase polarity, solute elution order and the effect of
increasing mobile phase polarity on the elution time.
(4)
Page 5 of 8
 |
6 Page 6 |
▲back to top |

4.4 Provide the reason why conductivity detectors used in lon-Exchange
chromatography (IEC) have significant background signal and explain how this
problem is minimized.
(4)
4.5 Explain the principle of electrophoresis (CE).
(4)
4.6 In the expression of the van Deemter equation: H = A + B/u + Cu
(a) Which term(s) is (are) not applicable to capillary electrophoresis? Explain your
answer.
(3)
(b) What is the direct implication of the observation made in (a) in terms of column
efficiency?
(2)
END
Page 6 of8
 |
7 Page 7 |
▲back to top |

Physical Constants
Gas constant
Boltzmann constant
Planck constant
Faraday constant
Avogadro constant
Speed of light in vacuum
Mole volume of an ideal gas
Elementary charge
Rest mass of electron
Rest mass of proton
Rest mass of neutron
Permitivity of vacuum
Gravitational acceleration
Conversion
1W
1J
Factors
1 cal
leV
1Latm
1 atm
1 bar
1L
1 Angstrom
1 micron (1)
1 Poise
1 ppm
Selected Formulae
e = top
= 0.5(w,+w,)
R
k
h
F
LorNa
c
Vin
e
Me
Mp
Mn
fo
g
= 8.315) K+ mol?
= 8.315 kPa dm? K? mol
= 8.315 Pam? K+ mol?
= 8.206 x 10% L atm K* mol?
= 1.381 x 10°73J K+
= 6.626 x 10 J s*
= 9.649 x 10*C mol?
=6.022 x 1073 mol?
= 2.998x 108 ms?
= 22.41 L mol (at 1 atm and 273.15 K)
= 22.71 L mol? (at 1 bar and 273.15 K)
= 1.602 x 107°C
= 9.109 x 10%kg
= 1.673 x 10’ kg
= 1.675 x 10°’ kg
= 8.854 x 10° C2 J4m? (or Fm?)
= 9.807 ms?
=1Js*
=0.2390cal=1Nm=1VC
= 1Pam?=1kg ms?
= 4.184J
= 1.602 x 10°79)
= 101.3 J
= 1.013 x 10° N m* = 1.013 x 10° Pa
= 760 mmHg
=1x10°Pa
= 10 m? =? 1 dm?
=1x107°m =0.1 nm = 100 pm
=10m=1 um
=0.1 Pas=0.1N sm?
= 1g g*=1mg kg
= 1 mgL? (dilute aqueous solutions only)
2Az.
wietw,
p,-YN
4
8 Ly k,
a
1+ B
Page 7 of 8
 |
8 Page 8 |
▲back to top |

k qg=—2= tn,B —t m
k,
Fam
2
N = 16] f
w
q =nF
MG =-nFE
I=E/R
E = E°—RT/nF In ([B]°/[A]*)
E (for ISE): Ecen = K + 0.05916/z log[A]
E = hv (or E = hc/A)
A=-logT=logPo/P and A=ebc
Vp Phat
— 6nNr
Page 8 of 8





