 |
PCH602S - PHYSICAL CHEMISTRY - 2ND OPP -JULY 2023 |
 |
1 Page 1 |
▲back to top |

nAmlBIA unlVERSITY
OF SCIEnCE AnD TECHnOLOGY
FACULTYOF HEALTH,NATURALRESOURCESAND APPLIEDSCIENCES
SCHOOLOF NATURALAND APPLIEDSCIENCES
DEPARTMENTOF BIOLOGY,CHEMISTRYAND PHYSICS
QUALIFICATION:BACHELOROF SCIENCE
QUALIFICATION CODE: 07BOSC
COURSENAME: PHYSICALCHEMISTRY
SESSION:JULY 2023
DURATION: 3 HOURS
LEVEL:6
COURSECODE: PCH602S
PAPER:THEORY
MARKS: 100
SUPPLEMENTARY/SECOND OPPORTUNITYEXAMINATION QUESTION PAPER
EXAMINER(S) Prof Habauka M Kwaambwa
MODERATOR: Dr Euodia Hess
INSTRUCTIONS
1. Answer ALL the questions in Sections A and B.
2. Write clearly and neatly.
3. Number the answers clearly.
PERMISSIBLEMATERIALS
Non-programmable Calculators
ATTACHMENT
List of Useful Constants and Equation
THIS QUESTION PAPERCONSISTSOF 8 PAGES{Including this front page and list of useful
constants and equation as an attachment)
 |
2 Page 2 |
▲back to top |

SECTIONA: MULTIPLE CHOICEQUESTIONS
[20]
There are 20 questions in this section. Answer ALL questions by selecting the letter of the
correct answer. Each question carries 2 marks.
1. Which of the following statements is not true about the First Law ofThermodynamics?
A. Although energy may be converted from one form to another, it cannot be created
or destroyed.
B. When a chemical system changes from one state to another, the net transfer of
energy to its surroundings must be balanced by a corresponding change in the
internal energy of the system.
C. Energy U is a state function, meaning that its value is completely determined by
the thermodynamic state of the system.
D. Energy is not conserved in an isolated system, i.e. energy of an isolated system is
not constant.
E. There are 2 distinct ways to transport energy into or out of a closed system that is
not isolated, through heat, q, and work, w
2. For a reversible adiabatic process, change in entropy is
A. Maximum
B. Minimum
C. Zero
D. Negative
E. Unpredictable
3. Which of the following is a spontaneous process?
A. Freezing water at 40°C
B. Melting of ice at -273°C
C. Freezing of water at -24°C
D. Melting of ice at -24°C
E. Melting of ice at 100°C
4. Which of the following processes is most likely to lead to an increase in the entropy of
the system?
A. N2(g) + 3H2(g) 2NH3(g)
B. H2O(/) H2O(s)
C. SiH4(g)+ 2O2(g) SiO2(s)+ 2H2O(g)
D. NH4NO3(s) + H2O(/) NH4+(oq) + NO3·(oq)
E. NO(g) + O3(g) NO2(g)+ O2(g)
5. The equilibrium constant for a reaction is 0.48 at 25°C. What is the value of b.G0 at this
temperature?
A. 1.8 kJ
B. - 4.2 kJ
C. 1.5 x 102 kJ
D. 4.2 kJ
E. More information is needed
2
 |
3 Page 3 |
▲back to top |

6. Consider the reaction A(g) + B(g) 2C(g). For this reaction, t.H 0 = -116 kJ moI-1 and
the equilibrium constant Kpis 140 at 600 K. Calculate for this reaction (i) t.G0 and (ii)
t.S0 at 600 K.
A. (i) 24.7 kJmoI-1
(ii) -60.4 kJ K-1moI-1
B. (i) -60 kJmoI-1
(ii) -24.7 kJ K-1moI-1
C. (i) -24.7 kJmoI-1
(ii) -152 J K-1moI-1
D. (i) -24.7 kJmoI-1
(ii) -60.4 kJ K-1moI-1
E. None of the above
7. When the concentration of the reactants are measured in moldm- 3 and time in
seconds, what are the appropriate units for (i) rate and (ii) rate constant for the
reaction associated with rate law, Rate= k[A][B]?
A. (i) moldm- 3s-1
(ii) dm 3moI-1s-1
B. (i) moldm- 3s-1
(ii) dm 3moI-1s-1
C. (i) moldm- 3
(ii) dm 3moI-1s-1
D. (i) moldm- 3s-1
(ii) dm 6moI-1s-1
E. (i) moldm- 3s-1
(ii) dm-3moI-1s-1
8. When the concentration of A in a simple reaction A B was changed from 0.51
moldm- 3 to 1.03 moldm- 3, the half-life dropped from 150 seconds to 75 seconds at
25°C. What is the (i) order of the reaction and (ii) the value of the rate constant?
A. (i) n = 0 and
(ii) k = 1.70 x 10-3 moldm- 3s-1
B. (i) n = 1 and
C. (i) n = 2 and
D. (i) n =1 and
(ii) k = 1.31 x 10-2 s-1
(ii) k = 1.31 x 10-1 dm 3moI-1s-1
= (ii) k 4.62 x 10-3 s-1
E. None of the above
9. The reaction of a tertiary haloalkane (represented by RX below) with hydroxide ions
to follow the mechanism:
Step 1:
RX R++ x-
Step 2:
If Step 1 is found to be a slow step in the mechanism, which of the following
statements is true?
(i)
The reaction will be found to be first order with respect to RX.
(ii) The reaction has an intermediate represented by R+.
(iii) The reaction rate is independent of the concentration of the hydroxide ions.
A. (i) only
B. (ii) only
C. (iii) only
D. (i) and (ii) only
E. (i), (ii) and (iii)
3
 |
4 Page 4 |
▲back to top |

10. If the activation energy in the forward direction of an elementary step is 52 kJ and the
activation energy in the reverse direction is 74 kJ, what is the energy of reaction, .1H,
for this step?
A. 22 kJ
B. - 22 kJ
C. 52 kJ
D. - 52 kJ
E. 126 kJ
SECTION B
[80]
There are FOUR questions in this section. Answer ALL Questions.
QUESTION 1
[16]
{a) Briefly state each of the following laws:
{4)
{i) Boyle's law
{ii) Hess's law
{iii) Zeroth law of thermodynamics
(iv) Kirchhoff's law or equation
{b) State whether q, w, !1U and 11S are negative, zero or positive for cooling of an ideal
gas at constant volume.
{4)
{c) The statements below are all false. For each statement either correct it or state briefly
the reason for its being false.
(6)
{i) Charles' law states that the volume of a given amount of gas is directly
proportional to the absolute temperature on the Kelvin scale under any
conditions.
{ii) The total heat absorbed, q, for a cyclic process is equal to zero.
(iii) The Second Law of Thermodynamics states that the entropy of the system.
always increases during a spontaneous process.
(iv) Every closed system is isolated and every isolated system is closed.
{v) The work done for reversible process is greater than the work done for an
irreversible process.
=(au) {vi)
C
v
8T p
{d) For each of the following reactions, show or explain whether the heat evolved at
constant pressure {.1H) is smaller, larger than or the same as the heat evolved at
constant volume {.1U).
{2)
{i)
{ii) BaC/ 2 (s)+ F2
2CH 4 (g)
BaF2 (s)+ C/2 (g)
4
 |
5 Page 5 |
▲back to top |
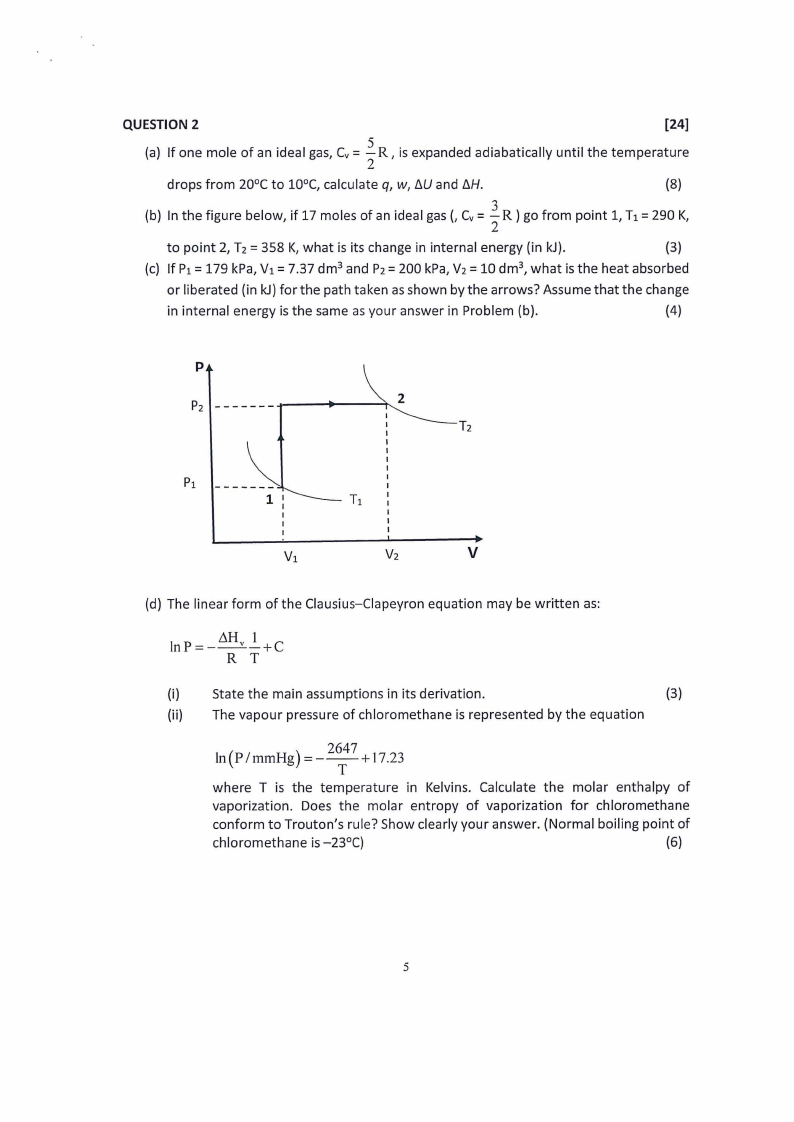
QUESTION 2
[24]
2. (a) If one mole of an ideal gas, Cv= R, is expanded adiabatically until the temperature
2
drops from 20°C to 10°C, calculate q, w, t:.U and t:.H.
(8)
(b) In the figure below, if 17 moles of an ideal gas(, Cv=IR) go from point 1, T1=290 K,
2
to point 2, T2 = 358 K, what is its change in internal energy (in kJ).
(3)
(c) If P1=179 kPa, V1=7.37 dm 3 and P2=200 kPa,V2=10 dm3, what is the heat absorbed
or liberated (in kJ)for the path taken as shown by the arrows? Assume that the change
in internal energy is the same as your answer in Problem (b).
(4)
p
V
(d) The linear form of the Clausius-Clapeyron equation may be written as:
InP =- ~Hv __!_+C
RT
(i) State the main assumptions in its derivation.
(3)
(ii) The vapour pressure of chloromethane is represented by the equation
ln(P/mmHg) =---2+647 17.23
T
where T is the temperature in Kelvins. Calculate the molar enthalpy of
vaporization. Does the molar entropy of vaporization for chloromethane
conform to Trouton's rule? Show clearly your answer. (Normal boiling point of
chloromethane is -23°C)
(6)
5
 |
6 Page 6 |
▲back to top |

QUESTION 3
[16]
(a) Given the standard enthalpy of formation and heat capacity data below, estimate the
enthalpy change at 100°C for the gas phase reaction: 3C2H2 C6H6
(8)
Species
Hr,298 /kJmol-
1
C2H2
226.7
C5H5
82.9
cp(c2 H2 )= 30.7 + 5.28 x 10-2T
cp(C6 H6 )= -1.7 + 32.5 x 10-2T
JK-1moI-1
JK-1moI-1
(b) Use the data below to calculate ~H 0 , ~S 0 , ~G 0 and Kpfor the reaction below at 298K.
CH3CH2CH2CH(3g) =; CH3CH(CH3)2(g)
(8)
Butane
2-Methylpropane
Compound
Butane
2-Methylpropane
t.Hr / kJn10I-1
-126 kJmoI-1
-125 kJmoI-1
S0 (298K)
270 JK-1moI-1
295 JK-1 moI-1
QUESTION 4
[24]
(a) The general expression for the integrated rate equations is of the form:
11
1
- -(-
n-1
[A-r---I
[A-r)'
= kt
for n ;t 1
State the two requirements in order to derive such equations.
(2)
(b) The reaction H2 + 12 2HI is first order with respect to [H2] and [12].When [H2] = 1
molL-1 and [bl= 2 molL-1, the following kinetic are observed.
d [HI]= 1.78 x 10-4 moIL-1s-1 at 556 Kand d [HI]= 0.2572 molL-1s-1 at 700 K.
dt
dt
Calculate the rate constant at each of the temperatures and evaluate the activation
energy and the Arrhenius pre-exponential factor, [Assume that the pre-exponential
factor is constant]
(8)
(c) The reverse reaction, i.e. 2HI H2+ Ii, has an activation energy of 183 kJmoI-1. Does
this make the reaction H2+ Ii -> 2HI exothermic or endothermic? Explain your answer
with a diagram of the energy profile of the reaction.
(6)
(d) Hydrogen peroxide, H2O2, decomposes in water by a first order kinetics process. A
0.156 moldm- 3 solution of H2O2 in water has an initial rate of 1.14 x 10-5 moldm 3s-1•
Calculate the rate constant, k, for the decomposition reaction and the half-life of the
decomposition.
(4)
6
 |
7 Page 7 |
▲back to top |

(e) Explain the meaning of intermediate and catalyst using the following mechanism,
which is thought to occur in the atmosphere:
(2)
NO+ 0 3
N0 2 + 0 2
0 + N0 2 ~NO+
02
(f) The gas decomposition of nitrous oxide (N20) is believed to occur via two elementary
steps
N 20~N
2 +0
N 20+0~N
2 +0 2
Experimentally, the rate is found to be rate= k[N20].
(i) Write the equation for the overall reaction.
(1)
(ii) What can you say about the relative rates of steps 1 and 2?
(1)
END OF EXAM QUESTIONS
7
 |
8 Page 8 |
▲back to top |

LIST OF USEFUL EQUATION AND CONSTANTS
Van der Waals eq!!. P= nRT
V-nb
n-?a = --R--T
v2
V-b
a
2
V
Universal Gas constant
Boltzmann's constant,
Planck's constant
Debye-Huckel's constant,
Faraday's constant
Mass of electron
Velocity of light
Avogadro's constant
1 electron volt (eV)
R=
k=
h=
A=
F=
me =
C
=
NA =
=
8.314 J K·1 mo1·1
1.381 X 10·23 J K"1
6.626 X 10-34 J S
0.509 (mol dm·3)112 or mo1·0·5kg0-5
96485 C mo1·1
9.109 X 10-31 kg
2.998 x 108 m s·1
6.022 X 1023
1.602 X 10-19 J
8





