 |
HTM811S - Heat Treatment of Metals 414 - 2nd Opp - June 2023 |
 |
1 Page 1 |
▲back to top |

nAmI BI AunIVER s ITY
OF SCIEn CE Ano TECHn OLOGY
FACULTYOF ENGINEERINGAND SPATIALSCIENCES
DEPARTMENT OF CIVIL, MINING AND PROCESSENGINEERING
QUALIFICATION: BACHELOR OF ENGINEERING IN METALLURGY
QUALIFICATION CODE: 08BEMT
LEVEL: 8
COURSE CODE: HTM811S
COURSE NAME: HEAT TREATMENT OF METALS 414
SESSION: June 2023
DURATION: 3 HOURS
PAPER: THEORY
MARKS: 100
EXAMINER(S)
SECONDOPPORTUNITY QUESTION PAPER
Mrs Jaquiline T. Kurasha
MODERATOR:
Prof Josias Van der Merwe
INSTRUCTIONS
1. Answer all questions.
2. Read all the questions carefully before answering.
3. Marks for each questions are indicated at the end of each question.
4. Please ensure that your writing is legible, neat and presentable.
PERMISSIBLEMATERIALS
1. Examination paper.
2. Non-programmable calculator.
THIS QUESTION PAPER CONSISTS OF 4 PAGES (Including this front page)
 |
2 Page 2 |
▲back to top |
Question 1 (25 marks]
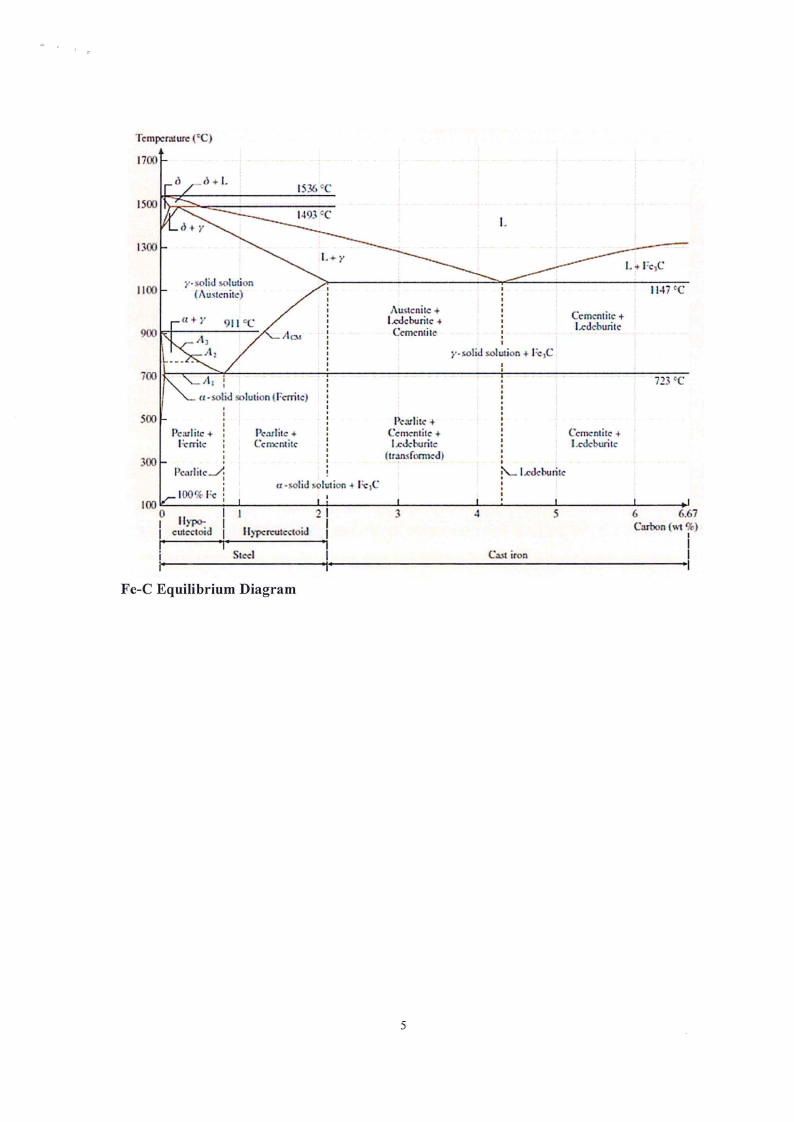
(a) Upon slow cooling a plain carbon steel 1080 contains 100% pearlite at room temperature.
Since pearlite is a eutectoid mixture of ferrite and cementite, calculate the weight
percentage of cementite and the weight percentage of ferrite in pearlite in a typical steel
1080. Make use of the Lever Rule and the Fe-C Equilibrium Diagram (see Appendix 1) [16]
(b) A Lab technician prepared three samples of steels: (i) a hypoeutectoid plain carbon steel
after slow cooling; (ii) a hypereutectoid plain carbon steel after slow cooling under the same
conditions; (iii) an austenitic stainless steel. Unfortunately, he failed to label the samples
properly. Suggest simple yet effective method to identify which sample is made of which
steel, with the aid of a magnet and a hardness tester. Please note that metallographic
microscope is out of operation.
[9]
Question 2 (25 marks]
A eutectoid plain carbon steel of 1080 type is commonly used for production of rails. It is
known that the railroads transporting mining products experience severe loading. In order to
produce rails with even higher hardness, wear resistance, and toughness, Japan Steel has
developed an advanced heat treatment schedule to form very fine pearlite in steel 1080. This
advanced schedule is featured in Figure Q2 together with two other treatments. With the aid
of Figure Q2 answer the following questions:
2
C..;) ..
i:.i
0..
E
Martcnsitc
V3
V1
V2
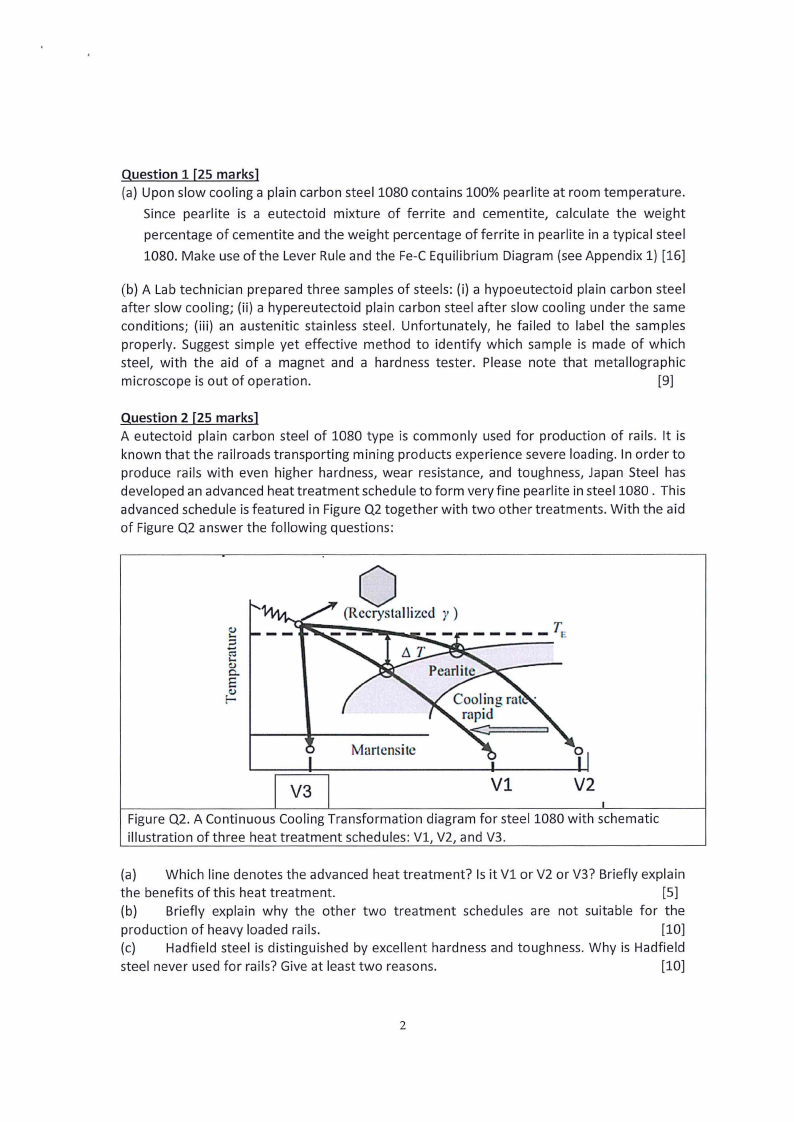
Figure Q2. A Continuous Cooling Transformation diagram for steel 1080 with schematic
illustration of three heat treatment schedules: Vl, V2, and V3.
(a) Which line denotes the advanced heat treatment? Is it Vl or V2 or V3? Briefly explain
the benefits of this heat treatment.
[5]
(b) Briefly explain why the other two treatment schedules are not suitable for the
production of heavy loaded rails.
[10]
(c) Hadfield steel is distinguished by excellent hardness and toughness. Why is Hadfield
steel never used for rails? Give at least two reasons.
[10]
2
 |
3 Page 3 |
▲back to top |
Question 3 [25 marks]
(a) Typical media used for quenching include air, brine (10% salt in water), water, and oil.
(ii) Rank the four media in order of the cooling rate from fastest to slowest. [5]
(iii) During quenching in liquid media, very often either the part being cooled, or
the bath is agitated. Explain why.
[5]
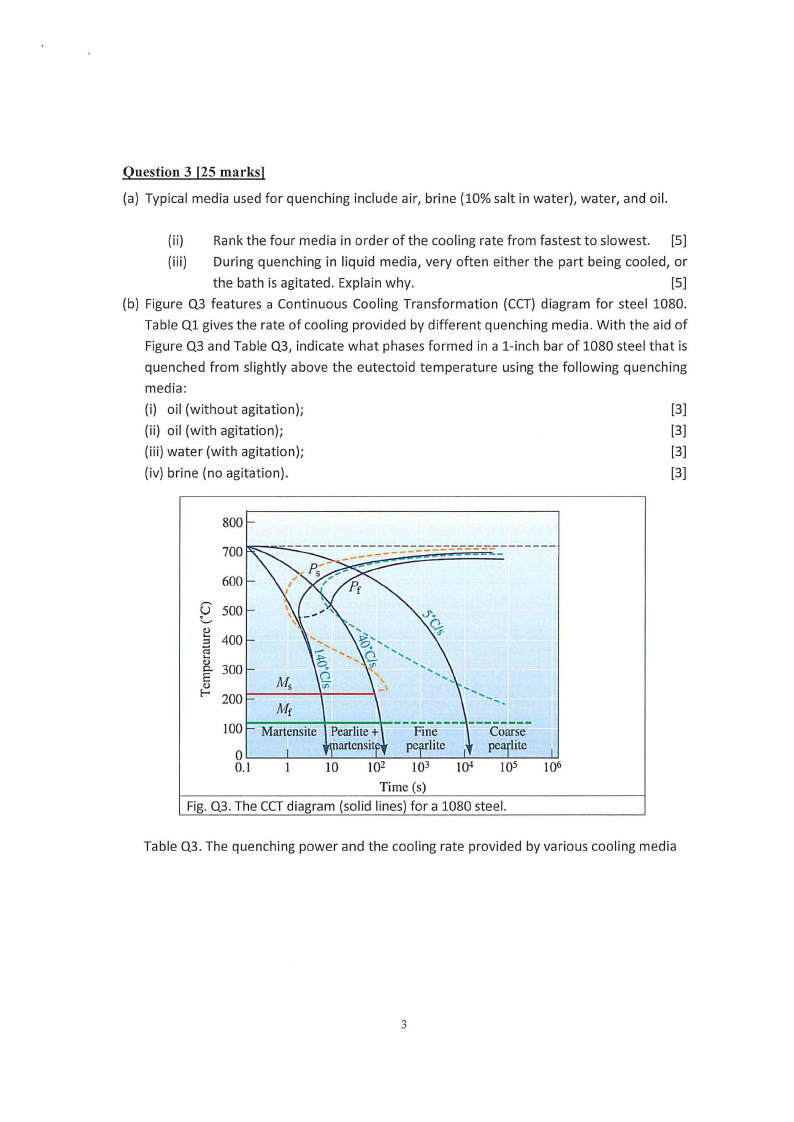
(b) Figure Q3 features a Continuous Cooling Transformation (CCT) diagram for steel 1080.
Table Q1 gives the rate of cooling provided by different quenching media. With the aid of
Figure Q3 and Table Q3, indicate what phases formed in a 1-inch bar of 1080 steel that is
quenched from slightly above the eutectoid temperature using the following quenching
media:
(i) oil (without agitation);
[3]
(ii) oil (with agitation);
[3]
(iii) water (with agitation);
[3]
(iv) brine (no agitation).
[3]
800
700
600
E soo
e::i 400
g_ 300
§
f-<
200 ------M--s-1
Mf
100 Martensite
00.~1 -~--~-~--1~0--~-1~0-2-~
Coarse
pea lite
104
1Q6
Time (s)
Fig. Q3. The CCTdiagram (solid lines) for a 1080 steel.
Table Q3. The quenching power and the cooling rate provided by various cooling media
3
 |
4 Page 4 |
▲back to top |
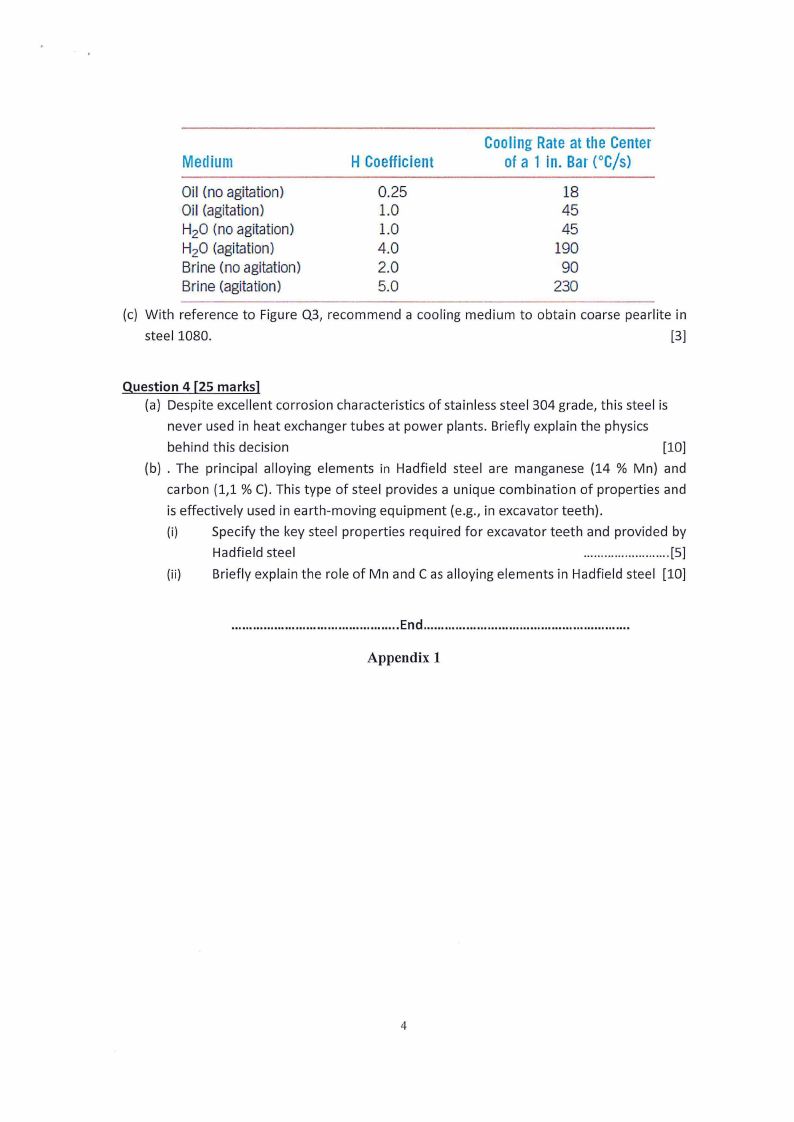
Medium
H Coefficient
CoolingRateat theCenter
ofa 1 in. Bar(°C/s)
Oil (no agitation)
0.25
18
Oil (agitation)
1.0
45
H2O (no agitation)
1.0
45
H2O (agitation)
4.0
190
Brine (no agitation)
2.0
90
Brine (agitation)
5.0
230
(c) With reference to Figure Q3, recommend a cooling medium to obtain coarse pearlite in
steel 1080.
[3]
Question 4 [25 marks]
(a) Despite excellent corrosion characteristics of stainless steel 304 grade, this steel is
never used in heat exchanger tubes at power plants. Briefly explain the physics
behind this decision
[10]
(b) . The principal alloying elements in Hadfield steel are manganese (14 % Mn} and
carbon (1,1 % C). This type of steel provides a unique combination of properties and
is effectively used in earth-moving equipment (e.g., in excavator teeth).
(i) Specify the key steel properties required for excavator teeth and provided by
Hadfield steel
........................(.5)
(ii) Briefly explain the role of Mn and C as alloying elements in Hadfield steel [10]
............................................... End ......................................................... .
Appendix 1
4
 |
5 Page 5 |
▲back to top |
Tcmpernture (0 C)
1700
1536cc
1500
L
1300
1100
:,--solid solution
(i\\w1cni1c)
i\\ustcnilc +
l.cdcburirc +
Cc:mc:n1i1c
;,-solid solution + Fc1C
Ccmrntilc +
Lcdcburilc
11-17'C
t\\ I
a-solid solution (ferrite)
5
Pc:ulirc +
Fc:rrilc
300
Pc.11li1.c.../.
I
100
0
I
I
llypo-
culccloid
Pc.:ulirc:+
Ccm::ntirc
P.:-:1.ditc+
Cc:mC'ntitc+
l..cdc:buritc
(t~fom,rd)
a -solid solution-+ Fc:1C
21
3
llypc:milcctoid
I
I
723 "C
Cc:mcntilc:+
l..cdcburitc
l.cdcburilc
5
C:ul iron
6
6.67
Camon (,,1 %)
I
I
I
Fe-C Equilibrium Diagram
5





