 |
AAC811S - ADVANCED ANALYTICAL METHOD AND CHEMOMETRICS - 2ND OPP - JULY 2022 |
 |
1 Page 1 |
▲back to top |

NAMIBIA UNIVERSITY
OF SCIENCE AND TECHNOLOGY
FACULTY OF HEALTH, APPLIED SCIENCES AND NATURAL RESOURCES
DEPARTMENT OF NATURAL AND APPLIED SCIENCES
QUALIFICATION: BACHELOR OF SCIENCE HONOURS
QUALIFICATION CODE: 08BOSH
LEVEL: 8
COURSE CODE: AAC811S
COURSE NAME: AANDDVANCCHEEDMOMAENATLRYITCISCAL METHOD
SESSION: JULY 2022
DURATION: 3 HOURS
PAPER: THEORY
MARKS: 100
SUPPLEMENTARY/SECOND OPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S) | DR JULIEN LUSILAO
MODERATOR: | PROF JAMES ABAH
INSTRUCTIONS
1. Answer ALL the questions in the answer book provided.
2. Write and number your answers clearly.
3. All written works MUST be done in blue or black ink.
PERMISSIBLE MATERIALS
Non-programmable Calculators
ATTACHMENTS
List of Useful Tables and formulas
THIS QUESTION PAPER CONSISTS OF 6 PAGES (Including this front page and attachments)
 |
2 Page 2 |
▲back to top |

Question 1
[20]
1.1 Name the different parts of the analytical strategy also known as the experimental
design.
(5)
1.2 A standard sample of pooled human blood serum contains 42.0 g of albumin/L.
A laboratory performs replicate determinations of the albumin concentration on
the same standard sample and obtains the following results (in g L“*): 42.2; 41.6;
42.0; 41.8; 42.6 and 39.0.
(a) Use appropriate statistics tests to assess whether the used method was affected
by systematic error (P = 0.05).
(5)
(b) It is suspected that the last replicate measurement is an outlier.
Use the recommended ISO test to confirm whether this is the case at P = 0.05.
(3)
(c) How does your finding in (b) affect the precision and accuracy of the results?
(3)
(d) What would be a more robust way of estimating the central tendency of the
obtained replicate measurements? Explain your choice.
(4)
Question 2
[30]
2.1 Sample containers used for the collection of solutions (liquids) samples are made
from glass or plastic.
(a) What are the common issues associated to glass containers?
(3)
(b) Clearly explain (with reaction if necessary) why glass containers are not that
recommended to collect solutions used for trace metals analysis.
(4)
(c) If glass bottles are the only available containers to collect liquid samples for heavy
metals analysis, how would you proceed during sampling to prevent the
underlined issue in (b) to happen?
(2)
2.2 Why is it important to reduce the size of solid particles present in a sample?
(4)
2.3 A quantitative analysis gives a mean concentration of 12.6 ppm for an analyte. The
method’s standard deviation (Smetn) is 1.1 ppm and the standard deviation for
sampling (Ssamp) is 2.1 ppm.
(a) What is the overall variance, S?, for the analysis?
(1)
 |
3 Page 3 |
▲back to top |
(b) By what percentage does the overall variance change if we improve Smeth by 10%
to 0.99 ppm?
(2)
(c) By what percentage does the overall variance change if we improve Ssamp by 10%
to 1.89 ppm?
(2)
(d) Briefly discuss the meaning/implication of your findings from (a) to (c).
(2)
2.4 Provide the main purpose of each of the following acids during a wet digestion
process
(a) Nitric acid
(1)
(b) Hydrochloric acid
(1)
(c) Aqua Regia
(1)
(d) Hydrofluoric acid
(1)
(e) Perchloric acid
(1)
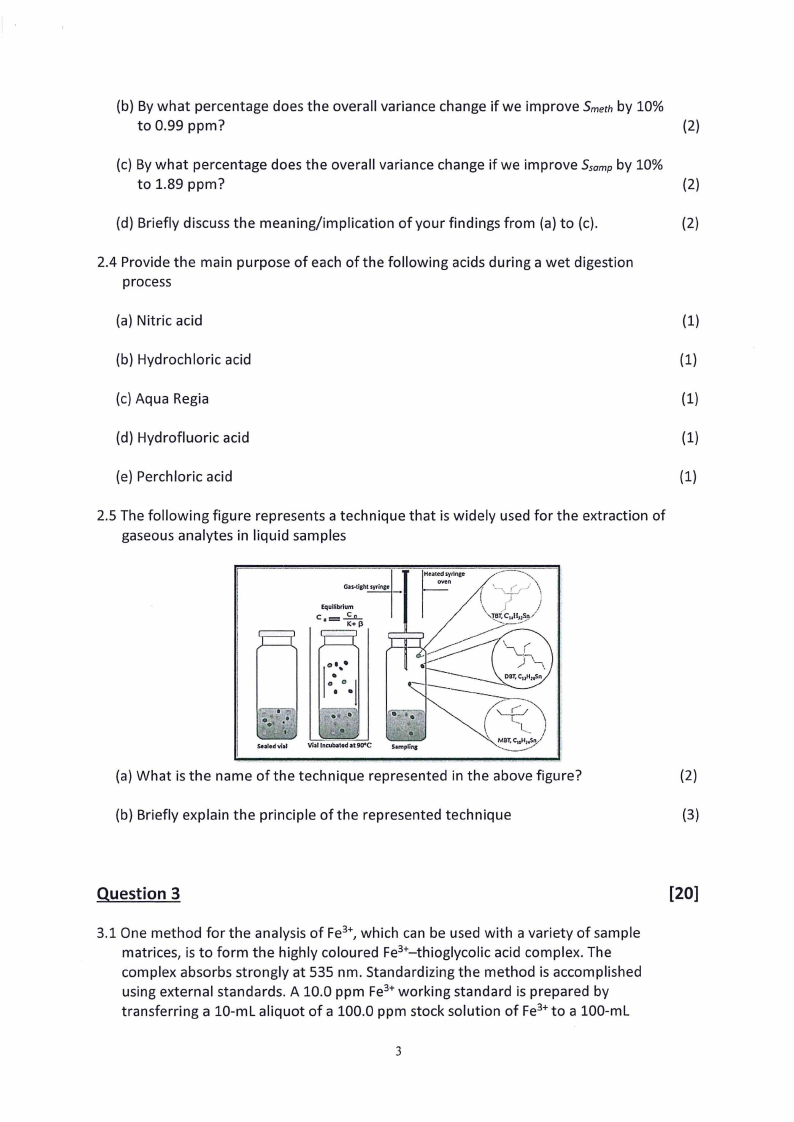
2.5 The following figure represents a technique that is widely used for the extraction of
gaseous analytes in liquid samples
Gas-tight syringe
Equilibrium
Heated syringe
Pe
;
{
(" aa |
Mat C“aLsLo/ } |'
(a) What is the name of the technique represented in the above figure?
(2)
(b) Briefly explain the principle of the represented technique
(3)
Question 3
[20]
3.1 One method for the analysis of Fe*, which can be used with a variety of sample
matrices, is to form the highly coloured Fe**—-thioglycolic acid complex. The
complex absorbs strongly at 535 nm. Standardizing the method is accomplished
using external standards. A 10.0 ppm Fe** working standard is prepared by
transferring a 10-mL aliquot of a 100.0 ppm stock solution of Fe* to a 100-mL
 |
4 Page 4 |
▲back to top |

volumetric flask and diluting to volume. Calibration standards of 1.0, 2.0, 3.0, 4.0,
and 5.0 ppm are prepared by transferring appropriate amounts of the 10.0 ppm
working solution into separate 50-mL volumetric flasks, each containing 5 mL of
thioglycolic acid, 2 mL of 20% w/v ammonium citrate, and 5 mL of 0.22 M NHs3.
After diluting to volume and mixing, the absorbances of the external standards
are measured against an appropriate blank. Samples are prepared for analysis by
taking a portion known to contain approximately 0.1 g of Fe**, dissolving in a
minimum amount of HNO3 and diluting to volume in a 1-L volumetric flask.
A 1.00-mL aliquot of this solution is transferred to a 50-mL volumetric flask, along
with 5 mL of thioglycolic acid, 2 mL of 20% w/v ammonium citrate, and 5 mL of
0.22 M NH3 and diluted to volume. The absorbance of this solution is used to
determine the concentration of Fe* in the sample.
(a) What is an appropriate blank for this procedure?
(2)
(b) Ammonium citrate is added to prevent the precipitation of Al?*. What is the effect
on the reported concentration of iron in the sample if there is a trace impurity of
Fe?* in the ammonium citrate?
(2)
(c) Why does the procedure specify that the sample contains about 0.1 g of Fe?*?
(3)
3.2 (a) Define an internal standard.
(1)
(b) What is the basic principle of internal standardisation?
(2)
(c) When do you use an internal standard?
(3)
3.3 Many of the analytical methods used to determine the concentration of fibrinogen
in plasma are based on light scattering following its precipitation. Light scattering
is measured nephelometrically at a wavelength of 340 nm. Analysis of a set of
external calibration standards gives the following calibration equation
Is = -4.66 + 9907.63 x C
where Is is the intensity of scattered light and C is the concentration of fibrinogen in
g/L. A9.0-mL sample of plasma was collected from a patient and mixed with 1.0 mL
of an anticoagulating agent. A 1.0-mL aliquot of this solution was then diluted to
250 mL in a volumetric flask. Analysis of the resulting solution gave a scattering
intensity of 44.70. What is the concentration of fibrinogen, in gram per liter, in the
plasma sample?
(4)
3.4 Give three disadvantages of the isotope dilution method.
(3)
Question 4
[30]
4.1 The following diagram describes different spectrometric techniques labelled A to D.





