 |
ENC702S - ENVIRONMENTAL CHEMISTRY - 1ST OPP - NOV 2022 |
 |
1 Page 1 |
▲back to top |
r
n
nAm I BI A u n IVE RS ITV
OF SCIEnCE Ano TECHnOLOGY
FACULTYOF HEALTH,NATURAL RESOURCESAND APPLIEDSCIENCES
DEPARTMENT OF NATURAL AND APPLIED SCIENCES
QUALIFICATION: BACHELOR OF SCIENCE
QUALIFICATION CODE: 07BOSC
LEVEL: 7
COURSE NAME: ENVIRONMENTAL CHEMISTRY COURSE CODE: ENC702S
SESSION: NOVEMBER 2022
DURATION: 3 HOURS
PAPER: THEORY
MARKS: 100
FIRSTOPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S) Dr JULIEN LUSILAO
MODERATOR: Dr JAMES ABAH
INSTRUCTIONS
1. Answer ALL the questions in the answer book provided.
2. Write and number your answers clearly.
3. All written work MUST be done in blue or black ink.
PERMISSIBLEMATERIALS
Non-programmable Calculators
THIS QUESTION PAPER CONSISTS OF 4 PAGES {Including this front page)
 |
2 Page 2 |
▲back to top |
Question 1
[20]
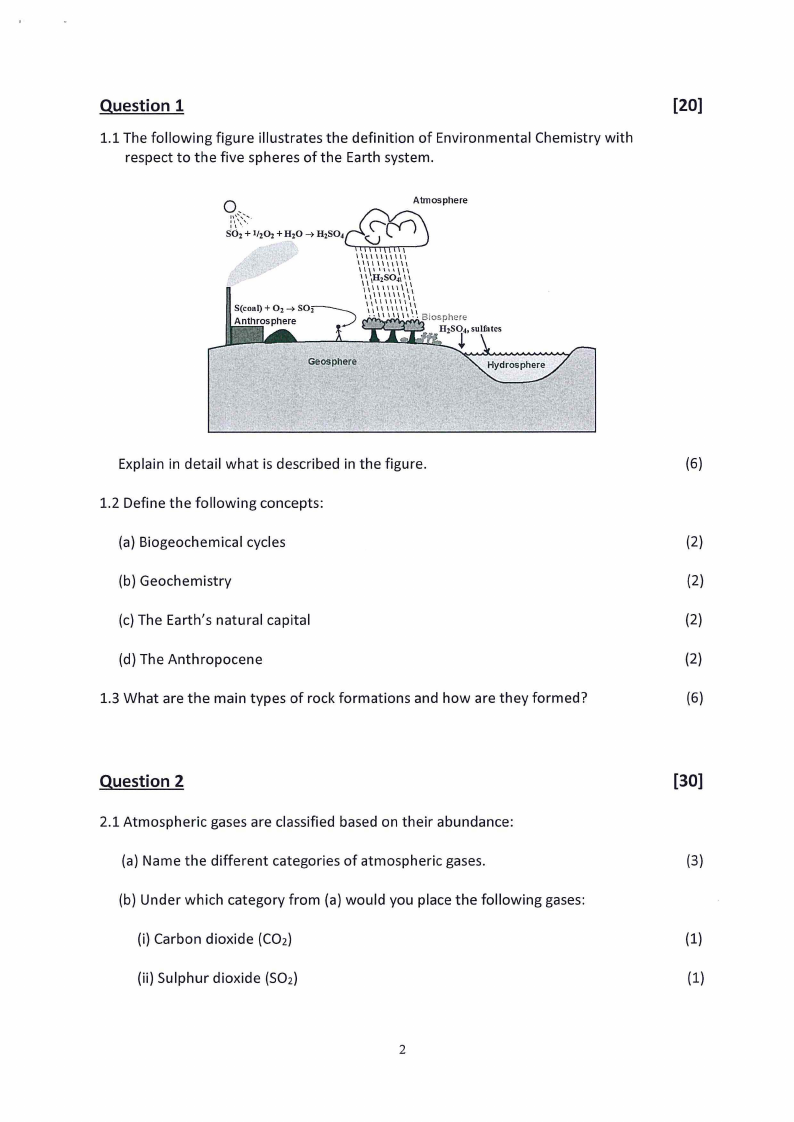
1.1 The following figure illustrates the definition of Environmental Chemistry with
respect to the five spheres of the Earth system.
Explain in detail what is described in the figure.
1.2 Define the following concepts:
(a) Biogeochemical cycles
(b) Geochemistry
(c) The Earth's natural capital
(d) The Anthropocene
1.3 What are the main types of rock formations and how are they formed?
Question 2
2.1 Atmospheric gases are classified based on their abundance:
(a) Name the different categories of atmospheric gases.
(b) Under which category from (a) would you place the following gases:
(i) Carbon dioxide (CO2)
(ii) Sulphur dioxide (502)
2
(6)
(2)
(2)
(2)
(2)
(6)
[30]
(3)
(1)
(1)
 |
3 Page 3 |
▲back to top |

(iii) Oxygen molecule {02}
(1)
2.2 Complete the following table about the main divisions of the atmospheric
compartment.
Region
Altitude (km) Temperature range (0 C}
(6)
2.3 (a) How would you define air pollution? Avoid the word "pollutant" in your
definition.
(2)
(b) Complete the following reactions related to the formation of inorganic particles:
(i) 3FeS2+ 802 _. _ + _
(2)
(ii) Cb+ 2NaOH _. __ + __ + H2O
(2)
2.4 (a) Differentiate between classical and photochemical smog.
(6)
(b) Nitrogen oxides (NOx) from car exhausts are known to be the main precursors of
photochemical smog and are, therefore, heavily regulated. The main approaches
used to control and limit their emissions are (i) control of combustion conditions
and (ii) removal from exhaust gas after combustion.
Describe how the two approaches in (i) and (ii) are practically applied.
(4)
Question 3
(30]
3.1 Water is transparent to visible and longer-wavelength fraction of ultraviolet light. it
has its maximum density as a liquid at 4°C (1 kg dm-3) and has the highest dielectric
constant of any common liquid (~ 80). What are the environmental significance or
effects of these three important properties of water?
(6)
3.2 Through the photosynthetic activity of algae, the pH of a water body was changed
from 7.0 to 10.0. If the water with alkalinity is 2.00 x 10-3 equivalents/liter,
calculate [CO2], [HCO3-], [COl-J, and [OH-] given Kai= 4.45 x 10-7 and
Ka2= 4.69 X 10-11.
(6)
3.3 What determines the upper and lower limits for the thermodynamic stability of
Water? Show the balanced redox reactions involved.
(6)
3
 |
4 Page 4 |
▲back to top |

3.4 Give the main role of the following microorganisms in aquatic environments.
(a) Algae
(2)
(b) Fungi
(2)
(c) Protozoa
(2)
3.5 Discuss the chemical, physical and biological characteristics of Acid Mine Drainage. (6)
Question 4
4.1 Write the chemical formula of the following minerals:
(a) Quartz (a silicate mineral)
(b) Magnetite (an oxide mineral)
(c) Calcite or limestone (a carbonate mineral)
(d) Pyrite, (a sulphide mineral)
(e) Gypsum (a sulphate mineral)
4.2 Briefly describe the soil composition.
[20]
(1)
(1)
(1)
(1)
(1)
(8)
4.3 (a) Match the soil or soil-solution constituent in (1) to (4) with the soil condition
described in (A) to (D) below:
Soil conditions
(A) "Cat clays" containing initially high
levels of pyrite, FeSz
(B) Soil in which biodegradation has not
occurred to a great extent
Soil constituents
(1) High Mn 2+ content in soil
(2) Excess W
(C) Waterlogged soil
(D) Soil, the fertility of which can be
improved by adding limestone
(3) High Wand S042- concentrations
(4) High organic content
(4)
(b) Explain the meaning of "internal processes" of natural hazards in the
geosphere.
(1)
(c) Provide two examples natural hazards caused by internal processes.
(2)
END
4





