 |
BPP702S - BIOCHEMISTRY BIOCHEMICAL PRINCIPLES AND PRACTICE - 1ST OPP - NOV 2022 |
 |
1 Page 1 |
▲back to top |

n Am I BI A u n IVE RS ITV
OF SCIEnCE Ano TECHnOLOGY
FACULTYOF HEALTH,NATURAL RESOURCESAND APPLIEDSCIENCES
DEPARTMENT OF NATURALAND APPLIEDSCIENCES
QUALIFICATION: BACHELOR OF SCIENCE(MAJOR/MINOR)
QUALIFICATION CODE: 07BOSH
LEVEL: 7
COURSENAME: BIOCHEMISTRY: BIOCHEMICAL COURSECODE: BPP702S
PRINCIPLESAND PRACTICE
SESSION:NOVEMBER 2022
DURATION: 3 HOURS
PAPER:THEORY
MARKS: 100
EXAMINER
FIRSTOPPORTUNITY QUESTION PAPER
DR LAMECH MWAPAGHA
MODERATOR ASSOC PROF PETRINA KAPEWANGOLO
INSTRUCTIONS
1. Answer ALL the questions.
2. Write clearly and neatly.
3. Number the answers clearly.
4. All written work MUST be done in BLUEor BLACKink.
PERMISSIBLEMATERIALS
None
THIS QUESTION PAPERCONSISTSOF FOUR (4) PAGES
(Including this front page)
1
 |
2 Page 2 |
▲back to top |
QUESTION 1
[11]
a) Distinguish between Positive and Negative Allosterism
(4)
b) The Department of natural and applied sciences has developed two enzymes that degrade
highly toxic compounds to non-toxic compounds. You have been tasked to degrade the
greatest amount of the toxic compound in the shortest amount of time.
The kinetic properties of the two enzymes are shown below:
Enzyme
A
B
Km
12mM
SmM
Vmax
700mM/sec
400mM/sec
I. At low concentrations of substrate which enzyme would be better to use based on the
information given above? (Which enzyme binds the substrate better).
Explain your answer.
(2)
II. At saturating concentrations of substrate, which enzyme would be better to use?
Explain your answer.
(2)
c) The kinetics of facilitated diffusion can be described by the Michaelis-Menten equation. Plot a
well labelled graph depicting both the facilitated and simple diffusion curves.
{3)
QUESTION 2
[12]
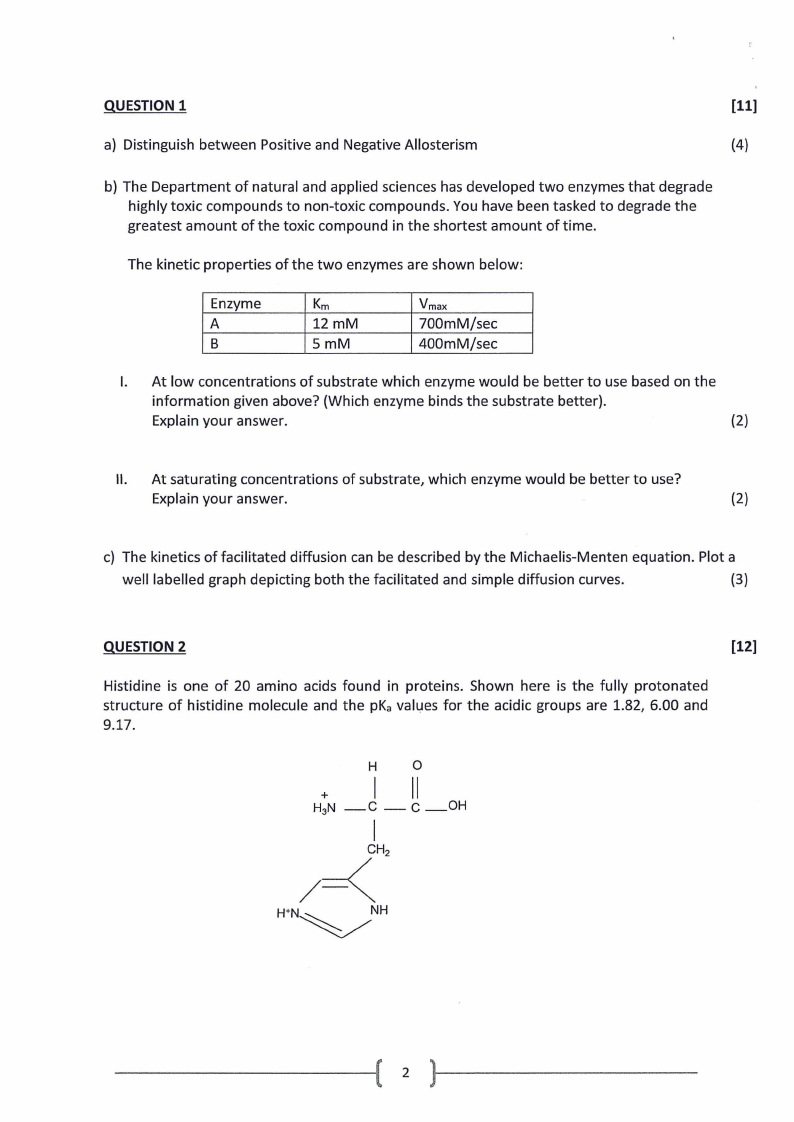
Histidine is one of 20 amino acids found in proteins. Shown here is the fully protonated
structure of histidine molecule and the pKa values for the acidic groups are 1.82, 6.00 and
9.17.
H
+I
H3N _c -
0
II
c_OH
I
/=<CH2
WN~NH
2
 |
3 Page 3 |
▲back to top |

a) What is the pl value of this amino acid? Show clearly how you arrive at the answer.
(6)
b) A buffer solution contains 0.25 M NH3 (Kb=1.8 xl0- 5 ) and 0.40 M NH4 CI. Calculate the pH of
this solution.
(3)
c) Calculate the pH of a solution 0.75 M lactic acid (Ka= 1.4 xl0- 4) and 0.25 M sodium lactate. (3)
QUESTION 3
[17]
a) The genetic code is the set of rules defining how the four-letter code of DNA is translated into
amino acids, which are the building blocks of proteins. Discuss THREE {3) characteristics of
the genetic code
(6)
b) Describe the mode of action of small interfering RNAs (siRNAs)
(3)
c) Briefly discuss the principles of metabolic pathways
(8)
QUESTION 4
[15]
a) Anaplerosis is the act of replenishing TCA cycle intermediates that have been extracted
for biosynthesis. Describe the anapleurotic reaction that aids is the formation of oxaloacetate
from pyruvate.
(5)
b) Oxidative phosphorylation is a process involving a flow of electrons through the electron
transport chain, a series of proteins and electron carriers within the mitochondrial
membrane. Briefly describe this process.
(6)
c) Using structural formulas, write the balanced chemical equation for the reactions where
FADH2is produced in the Kreb cycle.
(4)
3
 |
4 Page 4 |
▲back to top |
QUESTION 5
[14]
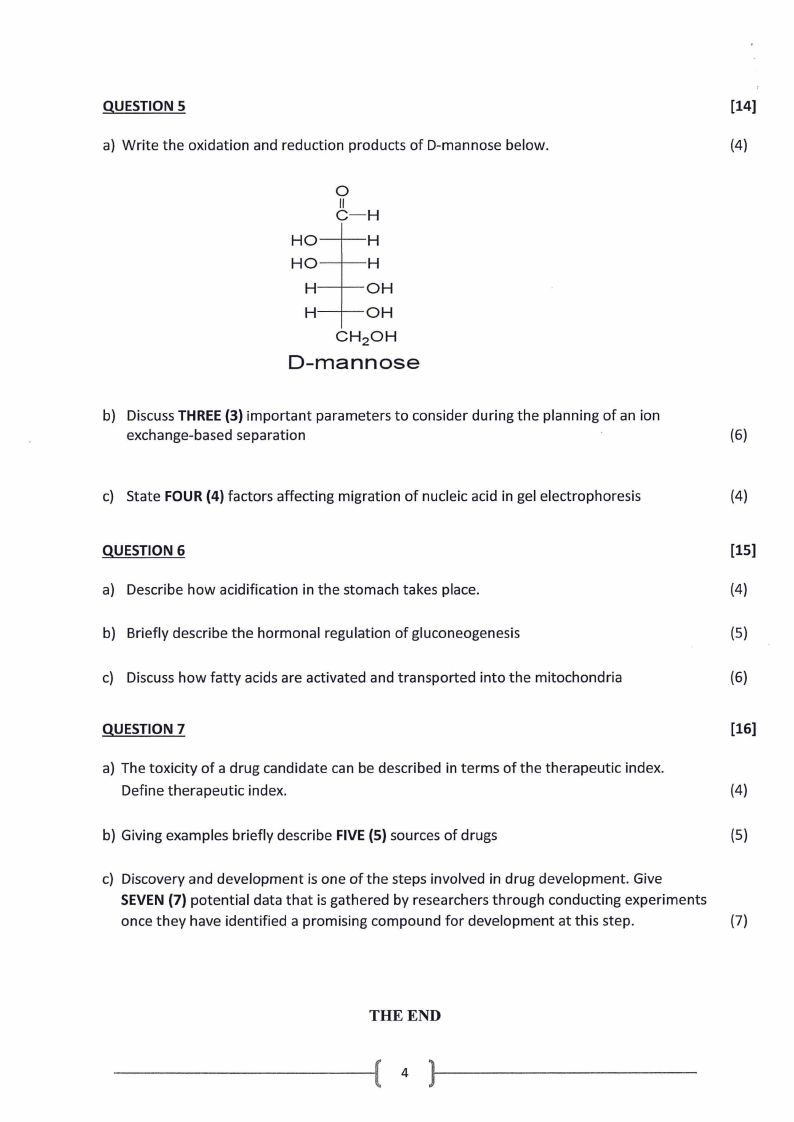
a) Write the oxidation and reduction products of D-mannose below.
(4)
0
II
C-H
HO--H
HO
H
H
OH
H
OH
CH 2 0H
D-mannose
b) Discuss THREE (3) important parameters to consider during the planning of an ion
exchange-based separation
(6)
c) State FOUR (4) factors affecting migration of nucleic acid in gel electrophoresis
(4)
QUESTION 6
[15]
a) Describe how acidification in the stomach takes place.
(4)
b) Briefly describe the hormonal regulation of gluconeogenesis
(5)
c) Discuss how fatty acids are activated and transported into the mitochondria
(6)
QUESTION 7
[16]
a) The toxicity of a drug candidate can be described in terms of the therapeutic index.
Define therapeutic index.
(4)
b) Giving examples briefly describe FIVE (5) sources of drugs
(5)
c) Discovery and development is one of the steps involved in drug development. Give
SEVEN (7) potential data that is gathered by researchers through conducting experiments
once they have identified a promising compound for development at this step.
(7)
THE END
4





