 |
MSC701S - MOLECULAR SPECTROSCOPY AND CHEMICAL SEPARTION METHODS - 2ND OPP - JULY 2023 |
 |
1 Page 1 |
▲back to top |

nAm I BI A un IVE RS ITV
OF SCIEnCE Ano TECHnOLOGY
FACULTYOF HEALTH,NATURALRESOURCESAND APPLIEDSCIENCES
SCHOOLOF NATURALAND APPLIEDSCIENCES
DEPARTMENTOF BIOLOGY,CHEMISTRYAND PHYSICS
QUALIFICATION: BACHELOR OF SCIENCE
QUALIFICATION CODE: 07BOSC
LEVEL: 7
COURSECODE: MSC701S
COURSENAME: MOLECULAR SPECTROSCOPYAND
CHEMICAL SEPARATION METHODS
SESSION:JULY 2023
DURATION: 3 HOURS
PAPER:THEORY
MARKS: 100
SUPPLEMENTARY/SECONDOPPORTUNITYEXAMINATION QUESTION PAPER
EXAMINER($) DR JULIEN LUSILAO
MODERATOR: A/PROF STEFAN LOUW
INSTRUCTIONS
1. Answer ALL the questions in the answer book provided.
2. Write and number your answers clearly.
3. All written work MUST be done in blue or black ink.
PERMISSIBLEMATERIALS
Non-programmable calculators
ATTACHMENTS
List of useful formulas and constants
THIS QUESTION PAPERCONSISTSOF 8 PAGES(Including this front page and attachments)
 |
2 Page 2 |
▲back to top |
Question 1
[25]
1.1 Define the following terms:
(a) Sensors
(2)
(b) A readout device
(2)
(c) Absorbance
(2)
1.2 It is critical in UV-Vis to measure the 100% transmittance (100%T). This measurement
is always carried out with a sample blank. Provide a clear explanation of the
relevance of 100%T in UV-Vis and the reason why a blank is used for that
measurement.
(5)
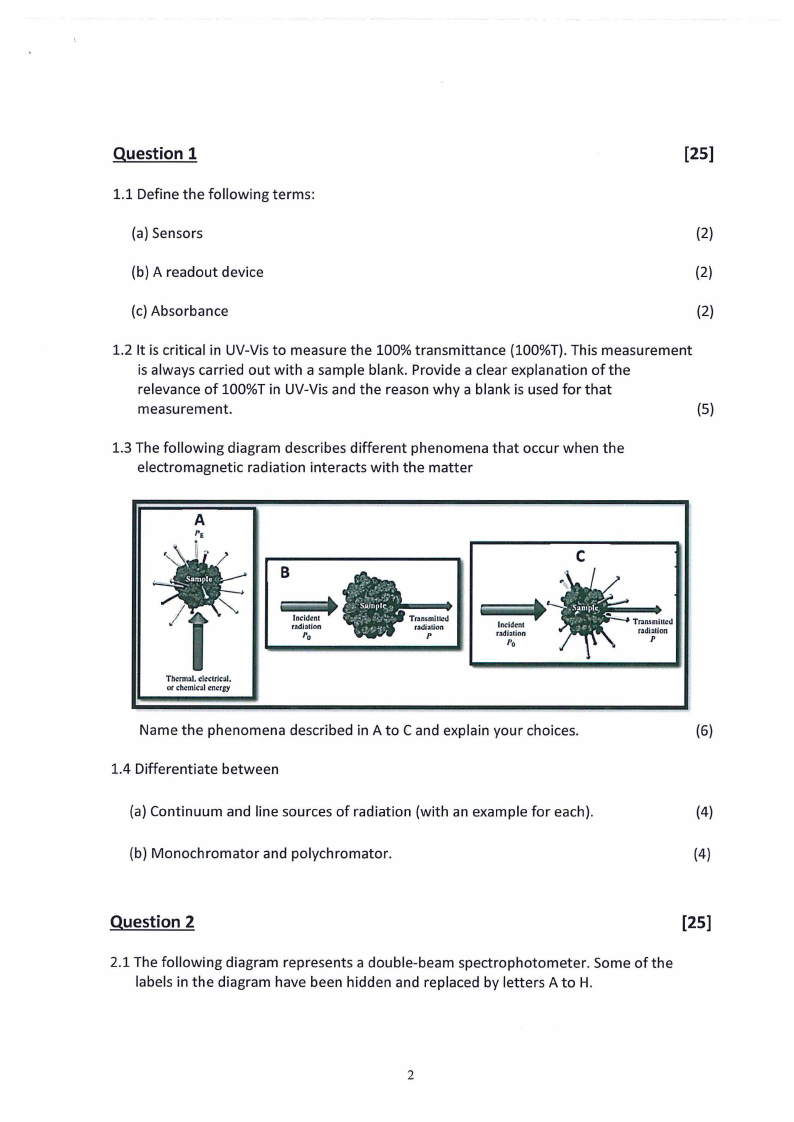
1.3 The following diagram describes different phenomena that occur when the
electromagnetic radiation interacts with the matter
A
C
B
Thcm1>Ic. lcc1rkal.
or chcmk•I energy
lncldcnl
r:1db1lon
Po
Tran,mlllcd
r:adi:llion
p
lnddcnl
radl>lion
Po
Name the phenomena described in A to C and explain your choices.
1.4 Differentiate between
(a) Continuum and line sources of radiation (with an example for each).
(b) Monochromator and polychromator.
Tr:msmlncd
rodi>llon
p
(6)
(4)
(4)
Question 2
[25]
2.1 The following diagram represents a double-beam spectrophotometer. Some of the
labels in the diagram have been hidden and replaced by letters A to H.
2
 |
3 Page 3 |
▲back to top |

H2
In this instrument, the device labelled "C" can be switched to the three positions
shown in Hl to H3. Provide the name of that device and clearly explain its role and
the purpose of these positions.
(5)
2.2 The following table shows the temperatures obtained with different combination of
fuels and oxidants in flame atomic absorption spectrometry (AAS).
Fuel
Natural gas
Natural gas
Hydrogen
Hydrogen
Acetylene
Acetylene
Acetylene
Oxidant
Air
Oxygen
Air
Oxygen
Air
Oxygen
Nitrous oxide
Temperature (0 C)
1700-1900
2700-2800
2000-2100
2550-2700
2100-2400
3050-3150
2600- 2800
(a) Which combination of the fuels above will produce better excitation of atoms?
Use the Boltzmann equation to support your choice.
(5)
(b) What analytical parameter(s) is (are) improved with a better atomization
efficiency?
(2)
(c) Besides the flame, name three other means of atomization used in AAS.
(3)
2.3 The burner assemblies of atomic absorption spectrometers (AAS) are known to
provide a long optical pathlength as well as a stable flame and they can also move
horizontally and vertically. Explain the importance of the underlined properties in
the statement above.
(4)
3
 |
4 Page 4 |
▲back to top |
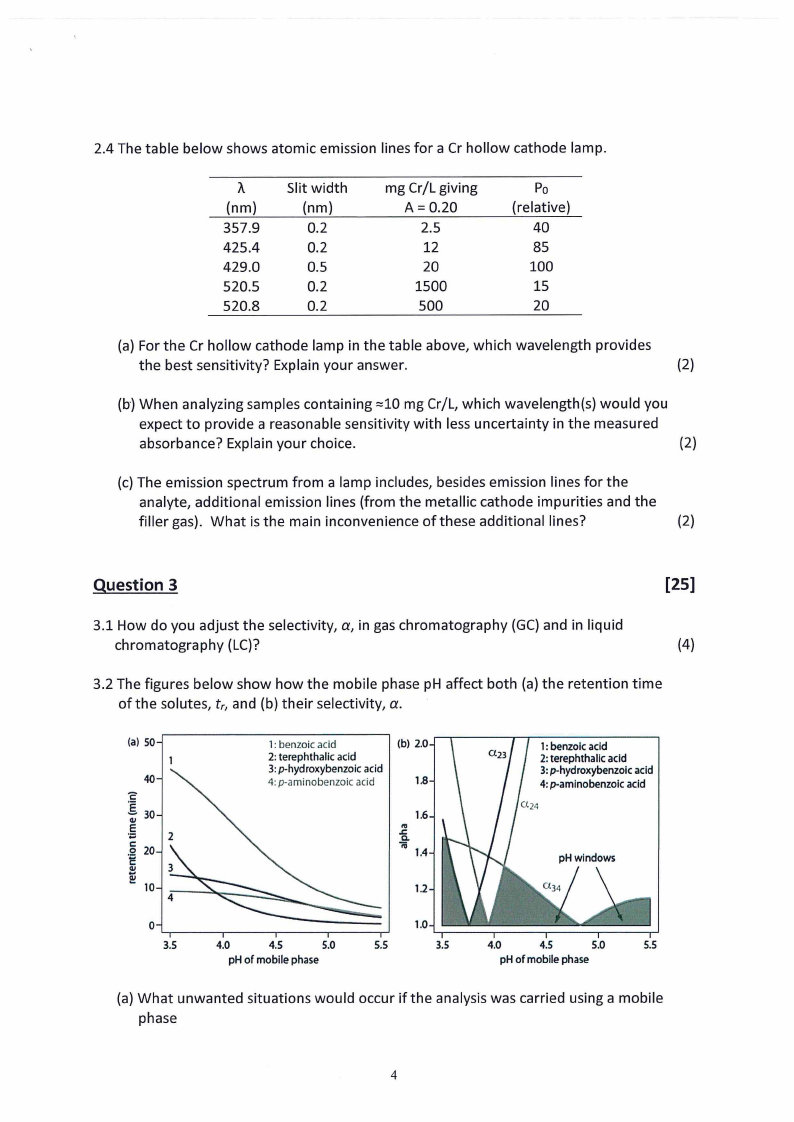
2.4 The table below shows atomic emission lines for a Cr hollow cathode lamp.
"A
(nm)
357.9
425.4
429.0
520.5
520.8
Slit width
(nm)
0.2
0.2
0.5
0.2
0.2
mg Cr/L giving
A= 0.20
2.5
12
20
1500
500
Po
(relative)
40
85
100
15
20
(a) For the Cr hollow cathode lamp in the table above, which wavelength provides
the best sensitivity? Explain your answer.
(2)
(b-)When analyzing samples containing :::10mg Cr/L, which wavelength(s) would you
expect to provide a reasonable sensitivity with less uncertainty in the measured
absorbance? Explain your choice.
(2)
(c) The emission spectrum from a lamp includes, besides emission lines for the
analyte, additional emission lines (from the metallic cathode impurities and the
filler gas). What is the main inconvenience of these additional lines?
(2)
Question 3
[25]
3.1 How do you adjust the selectivity, a, in gas chromatography (GC) and in liquid
chromatography (LC)?
(4)
3.2 The figures below show how the mobile phase pH affect both (a) the retention time
of the solutes, t,, and (b) their selectivity, a.
(a) 50
40
'i:
§ 30
QI
·E
.c§ 20
!!! 10
1: benzoic acid
2: terephthalic acid
3: p-hydroxybenzoic acid
4: p-aminobenzoic acid
(b) 2.0
1.8
1.6
."ac..'
iv 1.4
1.2
1: benzolc acid
2: terephthallc acid
3:p-hydroxybenzoicacid
4:p-amlnobenzoic acid
0
1.0
3.5
4.0
4.5
5.0
5.5
pH of mobile phase
3.5
4.0
4.5
5.0
5.5
pH of mobile phase
(a) What unwanted situations would occur if the analysis was carried using a mobile
phase
4
 |
5 Page 5 |
▲back to top |
(i) at pH between 5.0 and 5.5?
(1)
(ii) at pH 3.5?
(1)
(b) Figure (b) is also called a window diagram and is used to find the optimum
separation by plotting a for each pair of solutes. Using this figure, explain what
the optimum mobile phase pH would be to obtain the best chromatographic
separation of these 4 components.
(4)
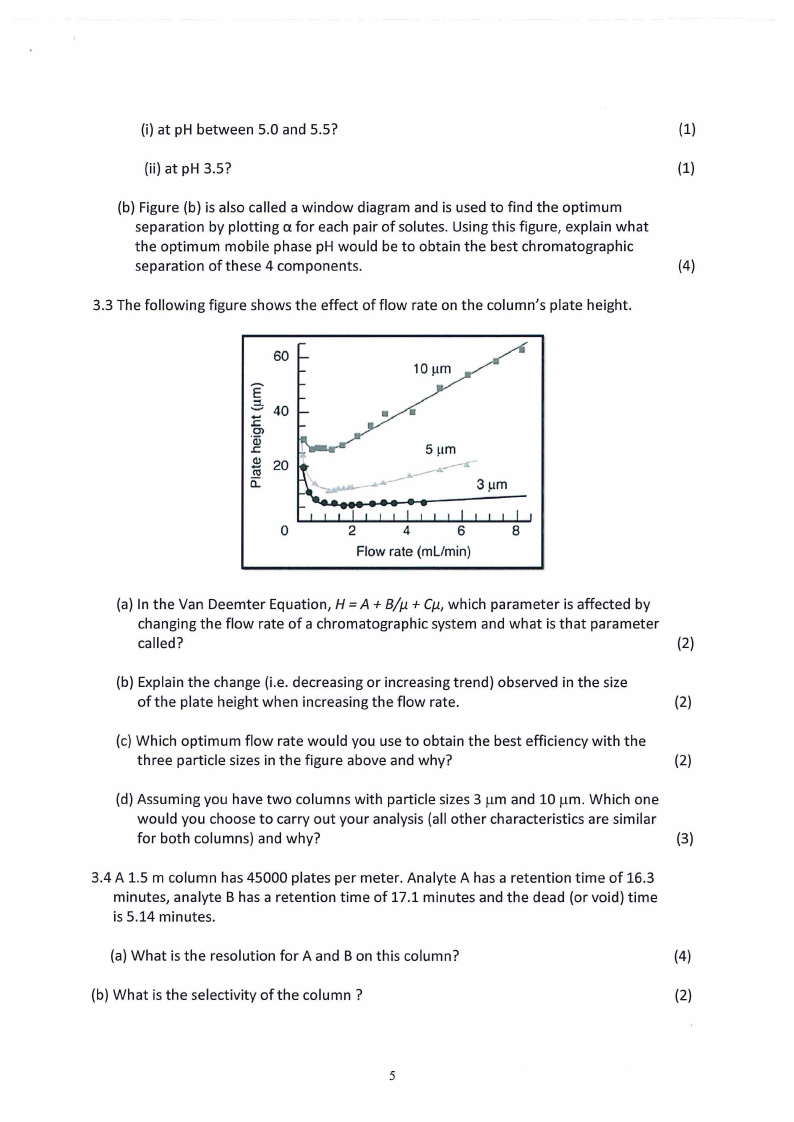
3.3 The following figure shows the effect of flow rate on the column's plate height.
60
--E
:::1. 40
.c
0)
·a3
a-:.c
(I}
rd
20
0
5 ~~Im-
3µm
2
4
6
8
Flow rate (ml/min)
(a) In the Van Deemter Equation, H = A + B/µ + Cµ, which parameter is affected by
changing the flow rate of a chromatographic system and what is that parameter
called?
(2)
(b) Explain the change (i.e. decreasing or increasing trend) observed in the size
of the plate height when increasing the flow rate.
(2)
(c) Which optimum flow rate would you use to obtain the best efficiency with the
three particle sizes in the figure above and why?
(2)
(d) Assuming you have two columns with particle sizes 3 µm and 10 µm. Which one
would you choose to carry out your analysis (all other characteristics are similar
for both columns) and why?
(3)
3.4 A 1.5 m column has 45000 plates per meter. Analyte A has a retention time of 16.3
minutes, analyte B has a retention time of 17.1 minutes and the dead (or void) time
is 5.14 minutes.
(a) What is the resolution for A and Bon this column?
(4)
(b) What is the selectivity of the column?
(2)
5
 |
6 Page 6 |
▲back to top |
Question 4
[25]
4.1 Briefly explain how solutes separate in a mixture when using the following
chromatographic techniques:
(a) Adsorption chromatography
(2)
(b) Partition chromatography
(2)
(c) Ion-exchange chromatography
(2)
(d) Size-exclusion chromatography
(2)
4.2 Name and briefly explain the different injection systems used in GC.
(6)
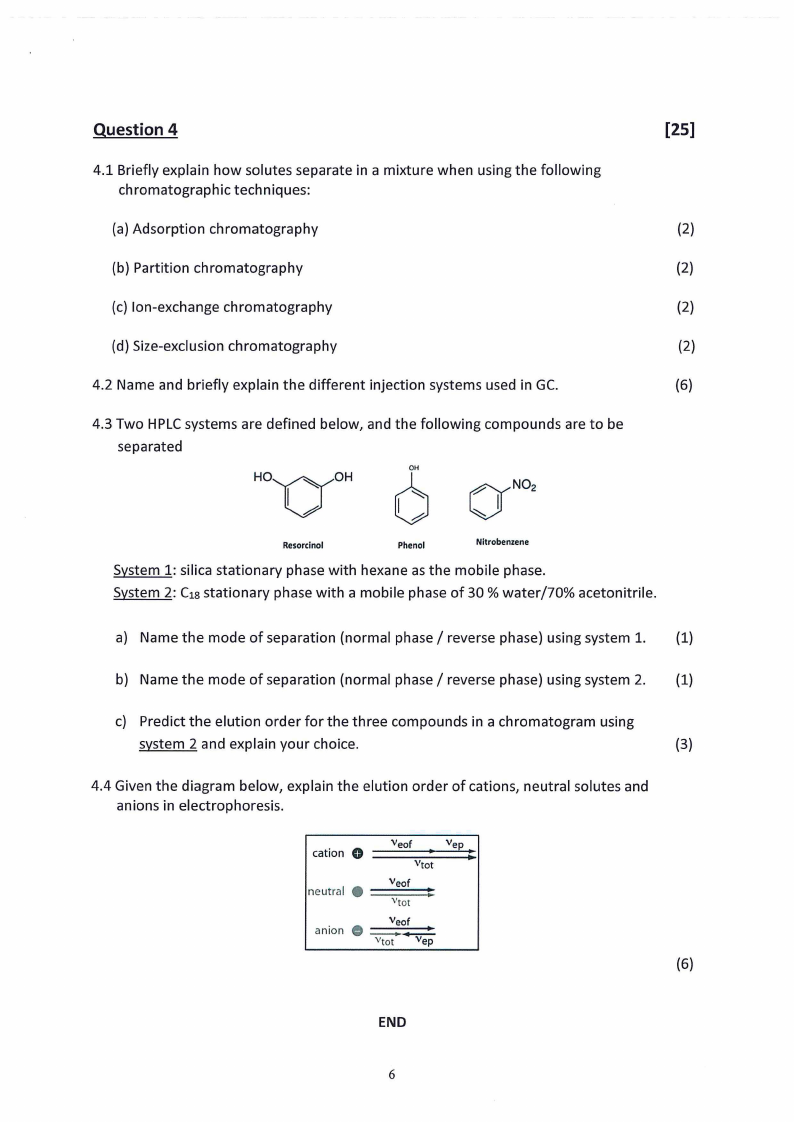
4.3 Two HPLCsystems are defined below, and the following compounds are to be
separated
OH
HOYYOH
V
6
Resorclnol
Phenol
Nitrobenzene
System 1: silica stationary phase with hexane as the mobile phase.
System 2: C1sstationary phase with a mobile phase of 30 % water/70% acetonitrile.
a) Name the mode of separation (normal phase/ reverse phase) using system 1. (1)
b) Name the mode of separation (normal phase/ reverse phase) using system 2. (1)
c) Predict the elution order for the three compounds in a chromatogram using
system 2 and explain your choice.
(3)
4.4 Given the diagram below, explain the elution order of cations, neutral solutes and
anions in electrophoresis.
cation 0
' Veof
Vee'
Vtot
e neutral
Veof
"tot
anion O _...V.,e.o,f._
"tot Vep
(6)
END
6
 |
7 Page 7 |
▲back to top |

Physical Constants
Gas constant
Boltzmann constant
Planck constant
Faraday constant
Avogadro constant
Speed of light in vacuum
Mole volume of an ideal gas
Elementary charge
Rest mass of electron
Rest mass of proton
Rest mass of neutron
Permitivity of vacuum
Gravitational acceleration
Conversion Factors
1W
1J
1 cal
1 eV
1 L atm
1 atm
1 bar
ll
1 Angstrom
1 micron(µ)
1 Poise
1 ppm
Selected Formulae
R
k
h
F
L or NA
c
Vm
e
me
mp
mn
Eo
g
= 8.315 J K-1 moI-1
= 8.315 kPa dm 3 K-1 moI-1
= 8.315 Pa m3 K-1 moI-1
= 8.206 x 10-2 Latm K-1 moI-1
= 1.381 X 10-23 J K-1
= 6.626 x 10-34 J s-1
= 9.649 X 104 C moI-1
= 6.022 x 1023 moI-1
= 2.998 x 108 m s-1
= 22.41 LmoI-1 (at 1 atm and 273.15 K)
= 22.71 LmoI-1 (at 1 bar and 273.15 K)
= 1.602 x 10-19 C
= 9.109 X 10-31 kg
= 1.673 x 10-27 kg
= 1.675 x 10-27 kg
= 8.854 X 10-12 C2 J-1 m-1 (or F m-1)
= 9.807 m s-2
= 1 J s-1
= 0.2390 cal = 1 N rn = 1 V C
= 1 Pa m3 =1 kg m2 s-2
= 4.184 J
= 1.602 X 10-19 J
= 101.3 J
= 1.013 x 105 N m-2 = 1.013 x 105 Pa
= 760 mmHg
= 1 x 105 Pa
= 10-3 m3 = 1 dm 3
= 1 x 10-10 m = 0.1 nm= 100 pm
= 10-6 m = 1 µm
= 0.1 Pas= 0.1 N sm-2
= 1 µg g-1 = 1 mg kg-1
= 1 mg L-1 (dilute aqueous solutions only)
X 1- m
I
k=
t t -t
t
r= r
m =-r
Xm
tr
tm
tm
7
 |
8 Page 8 |
▲back to top |

q = nF
dG =-nFE
I= E/R
E = E0 - RT/nF In [B]b/[A]a
E (for ISE): Ecel=l K + 0.05916/z log[A]
= = E hv (or E he/A)
A = -log T = log Po/P and A = Ebe
8





