 |
PCH602S - PHYSICAL CHEMISTRY - 1ST OPP - NOV 2022 |
 |
1 Page 1 |
▲back to top |

n Am I BIA u n IVER s ITY
OF SCIEnCE Ano TECHn OLOGY
FACULTYOF HEALTH,NATURAL RESOURCESAND APPLIEDSCIENCES
DEPARTMENT OF NATURAL AND APPLIED SCIENCES
QUALIFICATION: VARIOUS
QUALIFICATION CODE: VARIOUS
COURSE NAME: PHYSICALCHEMISTRY
SESSION: NOVEMBER 2022
DURATION: 3 HOURS
LEVEL: 6
COURSE CODE: PCH602S
PAPER: THEORY
MARKS: 100
EXAMINER(S)
FIRST OPPORTUNITY EXAMINATION QUESTION PAPER
Prof Habauka M Kwaambwa
MODERATOR: Dr Euodia Hess
INSTRUCTIONS
1. Answer ALL the questions in Sections A and B.
2. Write clearly and neatly.
3. Number the answers clearly.
PERMISSIBLE MATERIALS
Non-programmable Calculators
ATTACHMENT
List of Useful Constants and Equation
THIS QUESTION PAPER CONSISTS OF 8 PAGES (Including this front page and a list of useful
constants and equation as an attachment)
 |
2 Page 2 |
▲back to top |

SECTION A: MULTIPLE CHOICE QUESTIONS
[20)
There are 10 questions in this section. Choose the correct answer. Each question carries 2
marks.
1. An ideal gas at 27°C is heated at constant pressure until its volume is doubled. The
final temperature is:
A. 54°C
B. 327°C
C. 108°C
D. 654°C
E. 600°C
2. Which of the following is not an intensive property?
A. Pressure
B. Temperature
C. Density
D. Heat
E. Molar volume
3. For a reversible power cycle, the operating temperature limits are 800 Kand 300 K. It
takes in 400 kJ of heat. The unavailable work will be:
A. 250 kJ
B. 150 kJ
C. 120 kJ
D. 100 kJ
E. Zero
4. If [I.G0 < 0, then K is __ . If £1.0G> 0, then K is __ . If [I.G0 = 0, then K is __ .
A. > 1, < 1, = 1
B. < 1, > 1, = 1
C. < 0, > 0, =0
D. > 0, < 0, =0
E. < 1, > 1, =0
5. Which of the following is not one of the assumptions in the derivation of the Clausius-
Clapeyron equation?
A. Vgas>>> Vnquid
·B. Gas behaves as ideal gas, i.e. V = RT/P for 1 mole
C. [I.Hvaporisatisonindependent of temperature in a given range
D. Solid Liquid
E. None of the above
2
 |
3 Page 3 |
▲back to top |
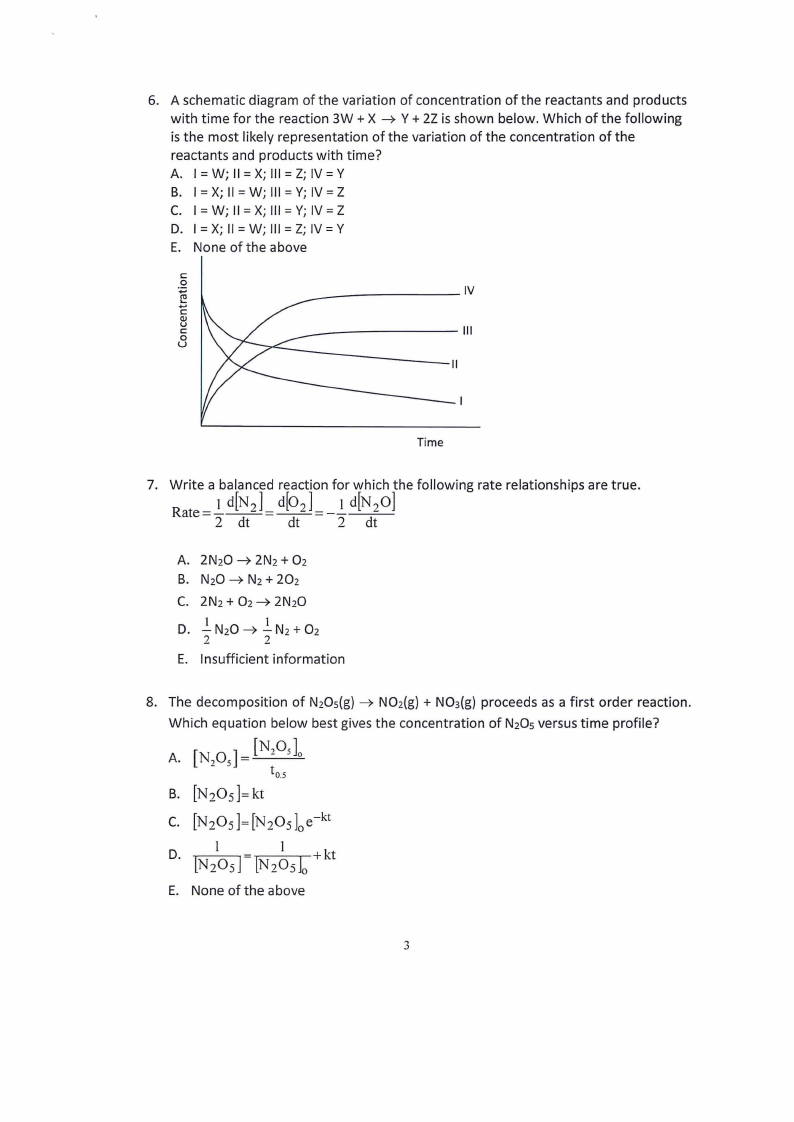
6. A schematic diagram of the variation of concentration of the reactants and products
with time for the reaction 3W + X Y + 2Z is shown below. Which of the following
is the most likely representation of the variation of the concentration of the
reactants and products with time?
A. I = W; II = X; Ill = Z; IV= Y
B. I = X; II = W; Ill = Y; IV= Z
C. I = W; II= X; Ill = Y; IV= Z
D. I = X; II= W; Ill = Z; IV= Y
E. None of the above
C:
:0;:;
________
IV
.....
C:
Qu J
C:
u0
---------111
Time
7. Write a balanced reaction for which the following rate relationships are true.
Rate=---21--d=[-d-N-t-?=]
d[o?]
dt
1
2
d[Nd?t- o]
A. 2N20 2N2 + 02
B. N20 N2+ 202
C. 2N2 + 02 2N20
D. -I N20 -I N2+ 02
2
2
E. Insufficient information
8. The decomposition of N20s(g) N02(g) + N03(g) proceeds as a first order reaction.
Which equation below best gives the concentration of N20s versus time profile?
A. [N20s] = [N20sL
to.s
B. [N20s]= kt
C. [N205 ]= [N205 ]0 e-kt
D. I
1 +kt
[N20sr [N20s] 0
E. None of the above
3
 |
4 Page 4 |
▲back to top |

9. The values for the change in enthalpy, L'lH,and the activation energy, EA,for a given
reaction are known. The value of fa for the reverse reaction equals
A. fa for the forward reaction
B. -(fa) for the forward reaction
C. the sum of -(L'lH) and EA
D. the sum of EAand L'lH
E. the difference between L'lHand EA
10. The balanced equation for the reaction of nitrogen dioxide and fluorine is
2N02 + F2 2N02F
The proposed mechanism is
Step 1:
N02 + F2 N02F + F
slow
Step 2:
F + N02 N02F
fast
Which of the following are correct?
(i) The mechanism supports an experimentally determined rate law of rate=
k[N02]2[F2]
(ii) Fis an intermediate
(iii) The reaction is first order with respect to Fi.
A. (i) only
B. (i) and (ii) only
C. (i) and (iii) only
D. (ii) and (iii) only
E. (i), (ii) and (iii)
SECTION B
[80]
There are FIVE questions in this section. Answer all Questions.
QUESTION 1
[12]
State whether each of the following statements is true or false. If false either correct it or
state briefly the reason for its being false.
(a) q = pctq = 0 and .6.T= pctT = 0, where q and Tis the heat absorbed and temperature,
respectively.
(2)
(b) The compressibility factor, Z > 1 for many gasesat high pressures is attributed to finite
size of gas molecules and repulsive forces.
(2)
(c) .6.Hcombus=tio.6n.Ucombustfioornthe combustion reaction
(2)
CH4(g)+ 202(g) C02(g) + 2H20(/)
(2)
(d) For the reaction 2C(g) + 02(g) 2C0(g), .6.H;eacti=on.6.H;(CO (g))
(2)
(e) For a perfect crystalline substance, S .,c = 0.
(2)
0
(au) (aG) (f)
=Sand
=Cv
(2)
8Tr
8Tv
4
 |
5 Page 5 |
▲back to top |
QUESTION 2
(a) State whether q, w, b.U, b.H and b.S are positive, negative or zero for reversible
adiabatic expansion of an ideal gas.
(5)
(b) A sample consisting of 2.00 mol argon (assume to behave as ideal gas) is expanded
reversibly and isothermally at 0°C from 22.4 dm3 to 44.8 dm3. For this process,
calculate q, w b.Uand b.H.
(8)
QUESTION 3
[13)
(a) Estimate the enthalpy change of formation for NH3(g) at 100°C given:
(3)
+f 1
...,
2 N 2 (g) H2 (g)
NH 3 (g), flH~ (25°C) = - 46.11 kJmol·1
Cp(N2,g) = 29.12 JK·1moI·1
Cp(H2,g) = 28.82 JK·1moI·1
Cp(NH3,g) = 35.06JK·1moI·1
(b) Calculate b.G0 for 1 mole of N2O4decomposition at 298 K, given Kp= 0.163. If b.S0 for
the reaction is 184.2 JK·1mol·1 at 298 K, calculate b.H0 at 298 K.
(3)
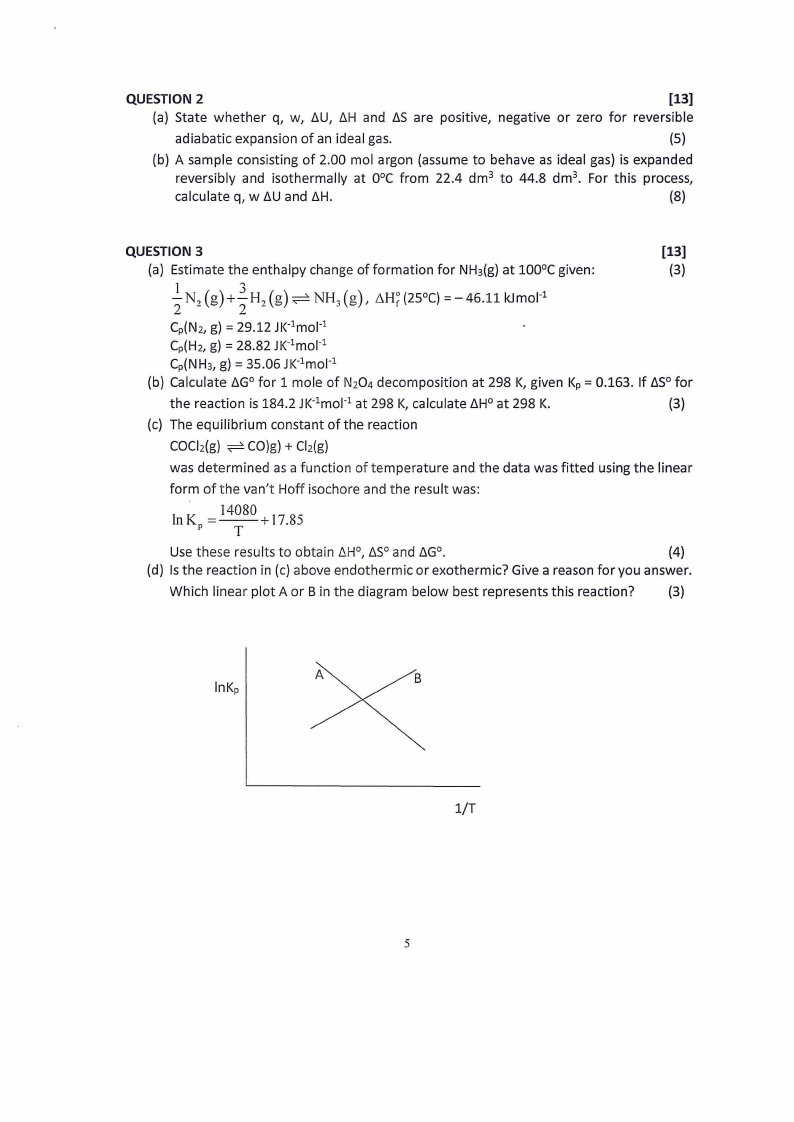
(c) The equilibrium constant of the reaction
COCl2(g) CO)g) + Cl2(g)
was determined as a function of temperature and the data was fitted using the linear
form of the van't Hoff isochore and the result was:
In KP= 14080 + 17.85
T
Use these results to obtain 6H0 , b.S0 and LiG0 •
(4)
(d) Is the reaction in (c) above endothermic or exothermic? Give a reason for you answer.
Which linear plot A or Bin the diagram below best represents this reaction?
(3)
A
lnKp
B
1/T
5
 |
6 Page 6 |
▲back to top |
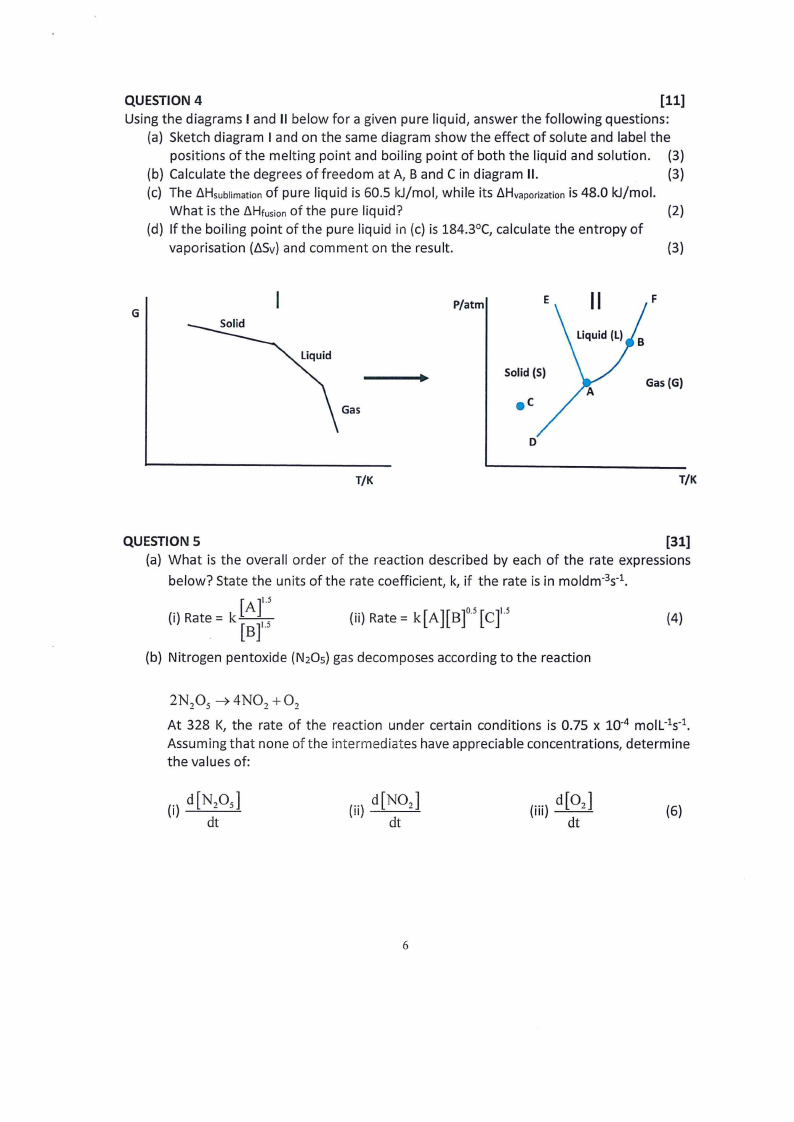
QUESTION 4
[11]
Using the diagrams I and II below for a given pure liquid, answer the following questions:
(a) Sketch diagram I and on the same diagram show the effect of solute and label the
positions of the melting point and boiling point of both the liquid and solution. (3)
(b) Calculate the degrees of freedom at A, Band C in diagram II.
(3)
(c) The LlHsublimatoiofnpure liquid is 60.5 kJ/mol, while its LlHvaporizatiiso4n8.0 kJ/mol.
What is the LlHtusioonf the pure liquid?
(2)
(d) If the boiling point of the pure liquid in (c) is 184.3°C, calculate the entropy of
vaporisation (LlSv)and comment on the result.
(3)
P/atm
F
G
Gas
T/K
Solid (S)
D
Gas (G)
T/K
QUESTION 5
[31]
(a) What is the overall order of the reaction described by each of the rate expressions
below? State the units of the rate coefficient, k, if the rate is in moldm·3s·1.
(i) Rate= k-[-A- ]1.s
(4)
. [Bf'
(b) Nitrogen pentoxide (N2Os)gas decomposes according to the reaction
2N 2 0 5
+0 2
At 328 K, the rate of the reaction under certain conditions is 0.75 x 10-4 molL·1s·1.
Assuming that none of the intermediates have appreciable concentrations, determine
the values of:
(I.I.)
d[N0,]
-
dt
(.1.1.)1--d [0 2 ]
(6)
dt
6
 |
7 Page 7 |
▲back to top |
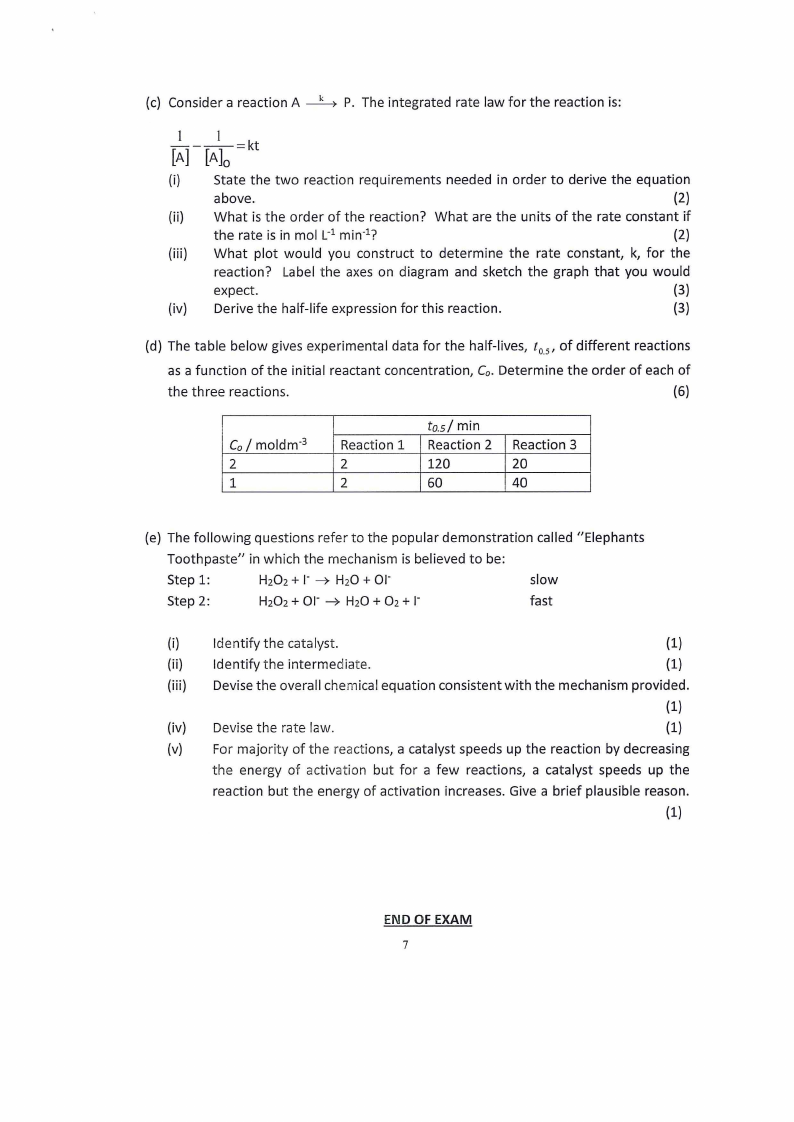
(c) Consider a reaction A
P. The integrated rate law for the reaction is:
_I __ l_=kt
[A] [A0]
(i) State the two reaction requirements needed in order to derive the equation
above.
(2)
(ii) What is the order of the reaction? What are the units of the rate constant if
the rate is in mol L-1 min-1?
(2)
(iii) What plot would you construct to determine the rate constant, k, for the
reaction? Label the axes on diagram and sketch the graph that you would
expect.
(3)
(iv) Derive the half-life expression for this reaction.
(3)
(d) The table below gives experimental data for the half-lives, t0.5 , of different reactions
as a function of the initial reactant concentration, Co. Determine the order of each of
the three reactions.
(6)
CoI moldm- 3
2
1
Reaction 1
2
2
to.sf min
Reaction 2
120
60
Reaction 3
20
40
(e) The following questions refer to the popular demonstration called "Elephants
Toothpaste" in which the mechanism is believed to be:
Step 1:
H202 + ,- H20 + oi-
slow
Step 2:
fast
(i) Identify the catalyst.
(1)
(ii) Identify the intermediate.
(1)
(iii) Devise the overall chemical equation consistent with the mechanism provided.
(1)
(iv) Devise the rate law.
(1)
(v) For majority of the reactions, a catalyst speeds up the reaction by decreasing
the energy of activation but for a few reactions, a catalyst speeds up the
reaction but the energy of activation increases. Give a brief plausible reason.
(1)
END OF EXAM
7
 |
8 Page 8 |
▲back to top |
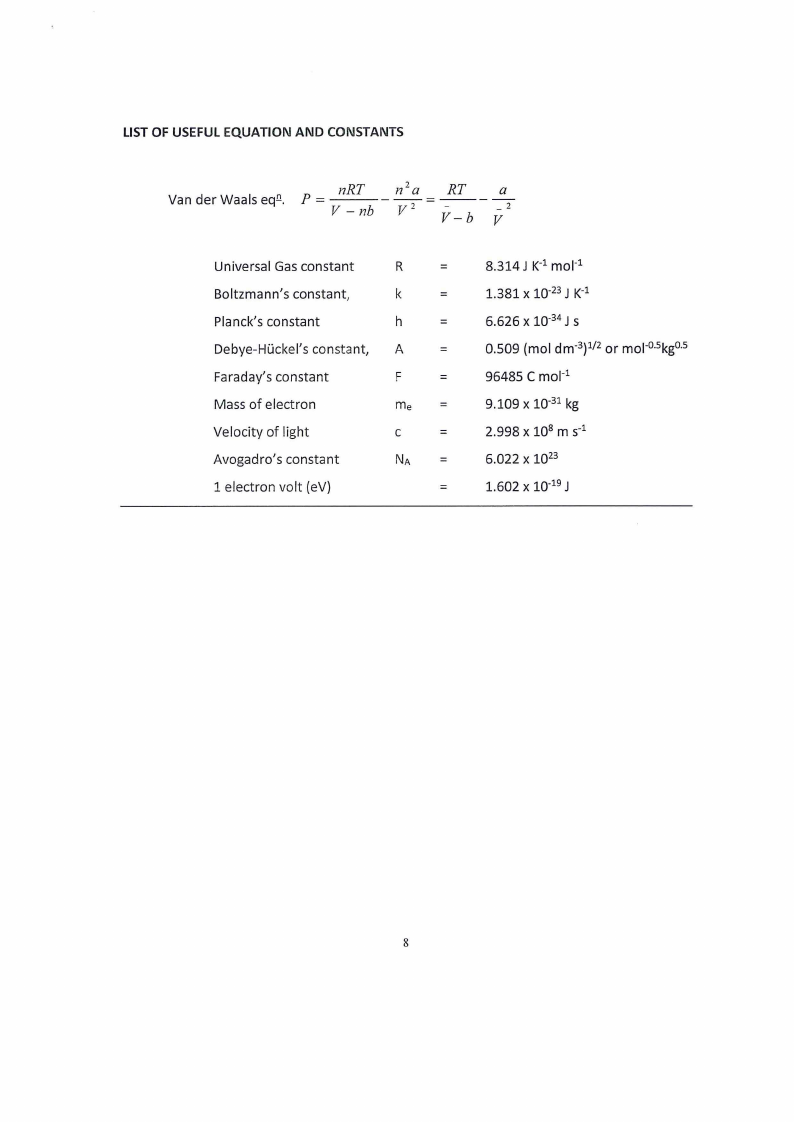
LIST OF USEFUL EQUATION AND CONSTANTS
nRT
Van der Waals eq.o.. P=
V -nb
---n-?a
v2
= --R--T
V-b
a
2
V
Universal Gas constant
Boltzmann's constant,
Planck's constant
Debye-Hi..ickel's constant,
Faraday's constant
Mass of electron
Velocity of light
Avogadro's constant
1 electron volt {eV)
R
=
k
=
h
=
A
=
F
=
me =
C
=
NA =
=
8.314 J K-1 mo1-1
1.381 X 10-23 J K-1
6.626 X 10-34 JS
0.509 (mol dm-3)112 or mo1-05 kg05
96485 C mo1-1
9.109 X 10-31 kg
2.998 x 108 m s-1
6.022 X 1023
1.602 X 10-19 J
8





