 |
CLC611S - CLINICAL CHEMISTRY 2A - 2ND OPP - JULY 2023 |
 |
1 Page 1 |
▲back to top |
nAm I BI A UnlVERS ITV
OF SCIEnCE Ano TECHnOLOGY
FACULTYOF HEALTH,APPLIEDSCIENCESAND NATURAL RESOURCES
SCHOOLOF HEALTH SCIENCES
DEPARTMENT OF CLINICALHEALTHSCIENCES
QUALIFICATION:BACHELOROF MEDICALLABORATORYSCIENCES
QUALIFICATIONCODE: 08BMLS
LEVEL: 6
COURSECODE: CLC611S
COURSENAME: CLINICAL CHEMISTRY 2A
SESSION:
JULY 23
PAPER:
THEORY
DURATION: 3 HOURS
MARKS:
105
SECONDOPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S)
MS CARAMIA DUNAISKI
MODERATOR:
DR MUNYARADZI MUKESI
INSTRUCTIONS
1. Answer ALL the questions.
2. Write clearly and neatly.
3. Number the answers clearly.
PERMISSIBLEMATERIALS
1. CALCULATOR
THIS QUESTION PAPERCONSISTSOF 5 PAGES(Including this front page)
 |
2 Page 2 |
▲back to top |

SECTION A [20]
[20]
QUESTION 1
[10]
Identify each of the following and only write the question number and
corresponding answer.
1.1 The mode of chromatographic separation based on competition between (1)
the sample and the mobile phase for adsorptive sites on a solid stationary
phase.
1.2 A formal recognition that a laboratory is competent to perform specified (1)
tests or measurements.
1.3 Identify the type if cuvette used in the visible range of the electromagnetic (1)
spectra.
1.4 Test measured using freezing point depression.
(1)
1.5 Photodetector which requires no external power source.
(1)
1.6 Type of water acceptable for most analytical requirements.
(1)
1.7 The electrode which tip is permeable only to CO2gas.
(1)
1.8 Express 45°F in K.
(1)
1.9 The strength of the bond between an antigen and an antibody.
(1)
1.10 Light scatter resulting from Antigen-Antibody complexes.
(1)
QUESTION 2
Define the following terms:
2.1 Zone electrophoresis
2.2 Specific gravity
2.3 Secondary standard
2.4 Detection limit
2.5 Proficiency test
[10]
(2)
(2)
(2)
(2)
(2)
Page2 of 5
 |
3 Page 3 |
▲back to top |
SECTION B
QUESTION 3
Enumerate the following. Please include all working in your answer.
MW: Na - 23, 0 - 16, Cl - 35.5, H - 1, C - 12
[35]
[20]
3.1 The analyte concentration in a sample is 1500 mg/dl. The sample was
diluted in a series as follows (table 1):
Table 1. Sample dilution protocol
Tube#
Tube 1
Tube 2
Tube 3
Tube 4
Tube 5
Dilution
1:5
1:2
1:4
1:5
1:10
3.1.1 What is the dilution factor in the final tube (tube 5)?
(2)
3.1.2 What is the concentration of sample in each tube?
(10)
3.2 How do you prepare 775ml of a 0.5% (w/v) solution of NaOH?
(2)
3.3 You need to make a 1:5 dilution of a solution. You need 10 ml of the
(2)
diluted solution. How much initial sample and diluent should you use?
3.4 If I leave 750 ml of 0.50 M NaCl solution uncovered on a windowsill and
(2)
150 ml of the solvent evaporates, what will the new concentration of the
NaCl solution be?
3.5 If I have 340 ml of a 0.5 M HCI solution, what will the concentration be if I (2)
add 560 ml more water to it?
QUESTION 4
[15]
Presented below are the results of daily quality control for serum amylase
measurement (figure 1). The control range is 60-90U/l (+/- 25D).
Page 3 of 5
 |
4 Page 4 |
▲back to top |
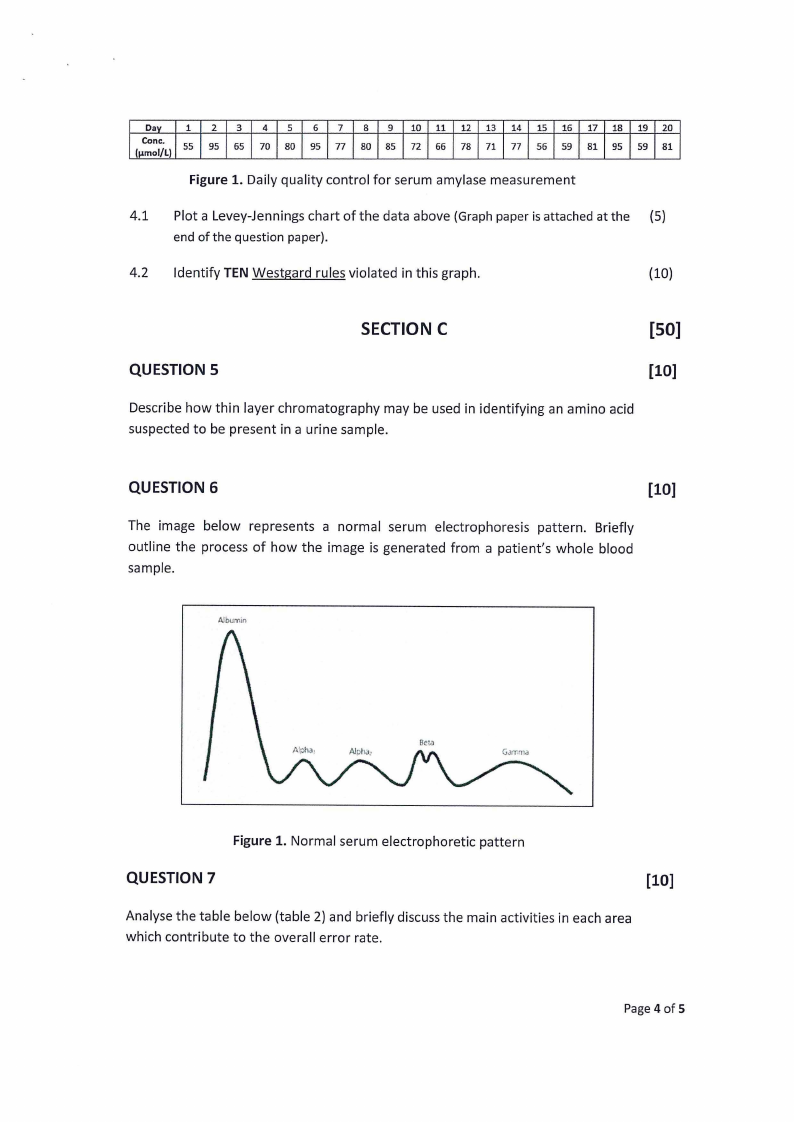
Dav 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
Cone.
h.1mol/L)
55
95
65
70
80
95
77
80
85
72
66
78
71
77
56
59
81
95
59
81
Figure 1. Daily quality control for serum amylase measurement
4.1 Plot a Levey-Jennings chart of the data above (Graphpaper is attached at the (5)
end of the question paper).
4.2 Identify TEN Westgard rules violated in this graph.
{10)
SECTION C
[SO]
QUESTION 5
[10]
Describe how thin layer chromatography may be used in identifying an amino acid
suspected to be present in a urine sample.
QUESTION 6
[10]
The image below represents a normal serum electrophoresis pattern. Briefly
outline the process of how the image is generated from a patient's whole blood
sample.
Albumin
Figure 1. Normal serum electrophoretic pattern
QUESTION 7
[10]
Analyse the table below (table 2) and briefly discuss the main activities in each area
which contribute to the overall error rate.
Page4 of 5
 |
5 Page 5 |
▲back to top |
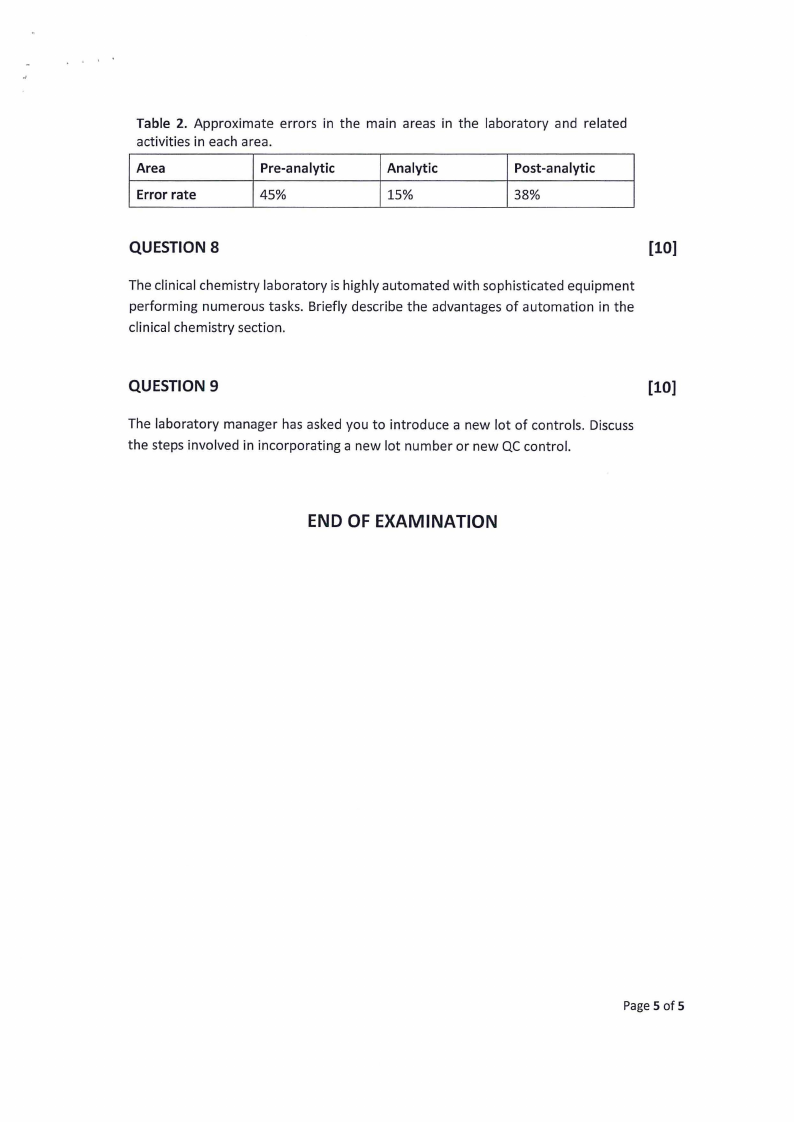
Table 2. Approximate errors in the main areas in the laboratory and related
activities in each area.
Area
Pre-analytic
Analytic
Post-analytic
Error rate
45%
15%
38%
QUESTION 8
[10]
The clinical chemistry laboratory is highly automated with sophisticated equipment
performing numerous tasks. Briefly describe the advantages of automation in the
clinical chemistry section.
QUESTION 9
[10]
The laboratory manager has asked you to introduce a new lot of controls. Discuss
the steps involved in incorporating a new lot number or new QC control.
END OF EXAMINATION
Page5 of 5





