|
AOC811S - ADVANCED ORGANIC CHEMISTRY - 1ST OPP - JUNE 2022 |
 |
1 Page 1 |
▲back to top |

NAMIBIA UNIVERSITY
OF SCIENCE AND TECHNOLOGY
FACULTY OF HEALTH, APPLIED SCIENCES AND NATURAL RESOURCES
DEPARTMENT OF NATURAL AND APPLIED SCIENCES
QUALIFICATION: BACHELOR OF SCIENCE HONOURS
QUALIFICATION CODE: O8BOSH
LEVEL: 8
COURSE CODE: AOC811S
COURSE NAME: ADVANCED ORGANIC CHEMISTRY
SESSION: JUNE 2022
PAPER: THEORY
DURATION: 3 HOURS
TOTAL MARKS: 100
FIRST OPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S) | DR. MARIUS MUTORWA
MODERATOR: | DR. RENATE HANS
INSTRUCTIONS
Answer ALL the questions.
Write clearly and neatly.
Number the answers clearly
All written work must be done in blue or black ink and sketches can
be done in pencil
5. No books, notes and other additional aids are allowed
PERMISSIBLE MATERIALS
Non-programmable Calculators
ATTACHMENTS
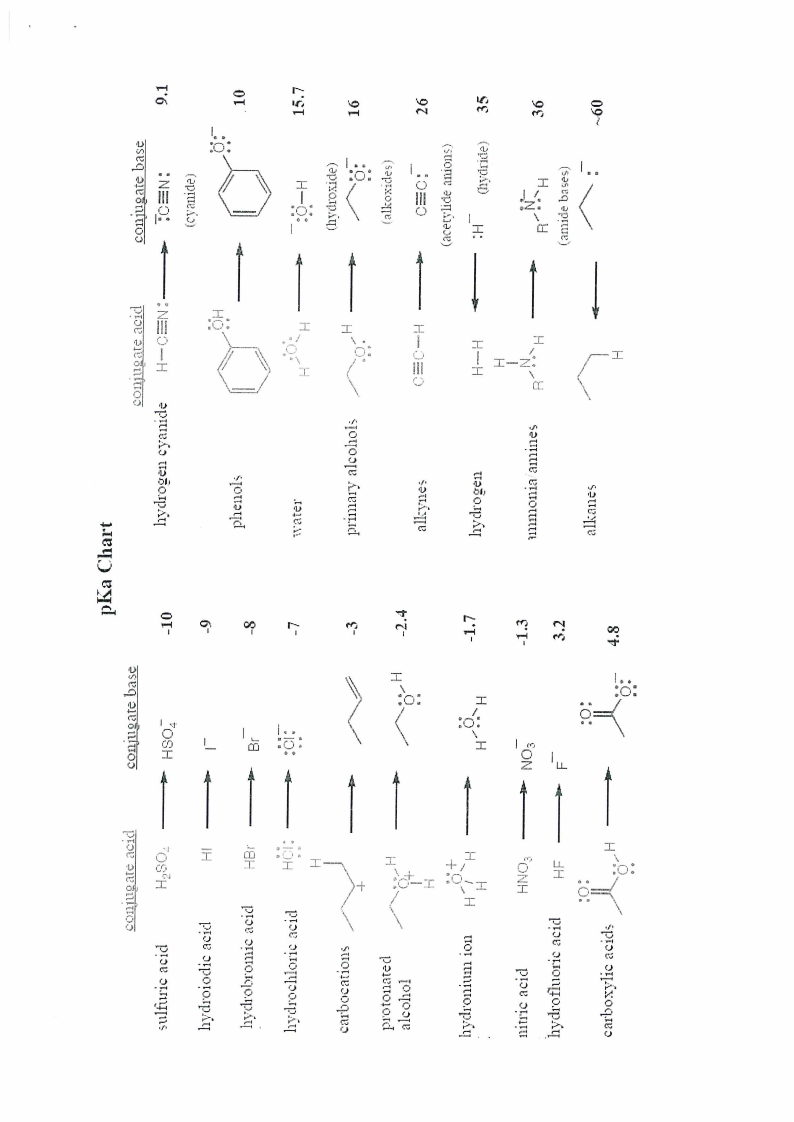
pKa Chart and Periodic Table
THIS QUESTION PAPER CONSISTS OF 7 PAGES
(Including this front page and attachments)
 |
2 Page 2 |
▲back to top |
QUESTION 1:
[20]
Question type: Enolates and Carbon Nucleophiles
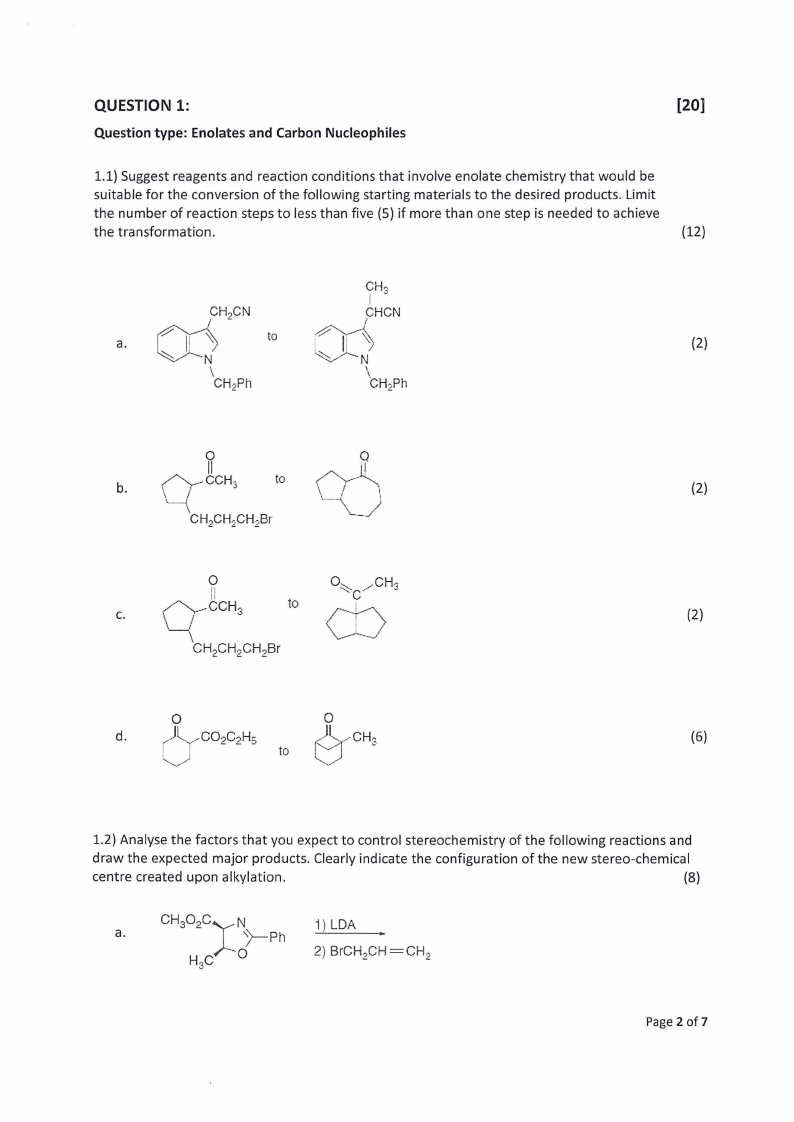
1.1) Suggest reagents and reaction conditions that involve enolate chemistry that would be
suitable for the conversion of the following starting materials to the desired products. Limit
the number of reaction steps to less than five (5) if more than one step is needed to achieve
the transformation.
(12)
CHs
CH,CN
CHCN
2 CLS &§ CYS
2)
SN
Se
‘CHP
‘CHPh
by -{
CH Oo|
2
s
t
4 oN= Qi
(2)
CH,CH.CH.Br
LY
0
Ox CHs
a ysC
oD to
|
(2)
if \\ CH,CH,CH,Br
ef j
O
O
d. JL c0sCoHs
Oy oH
(6)
4
‘0
Se
1.2) Analyse the factors that you expect to control stereochemistry of the following reactions and
draw the expected major products. Clearly indicate the configuration of the new stereo-chemical
centre created upon alkylation.
(8)
a.
CH302Ca1__N S—Ph
1) LDA
H,07 0
2) BrCH,CH=CH,
Page 2 of 7
 |
3 Page 3 |
▲back to top |
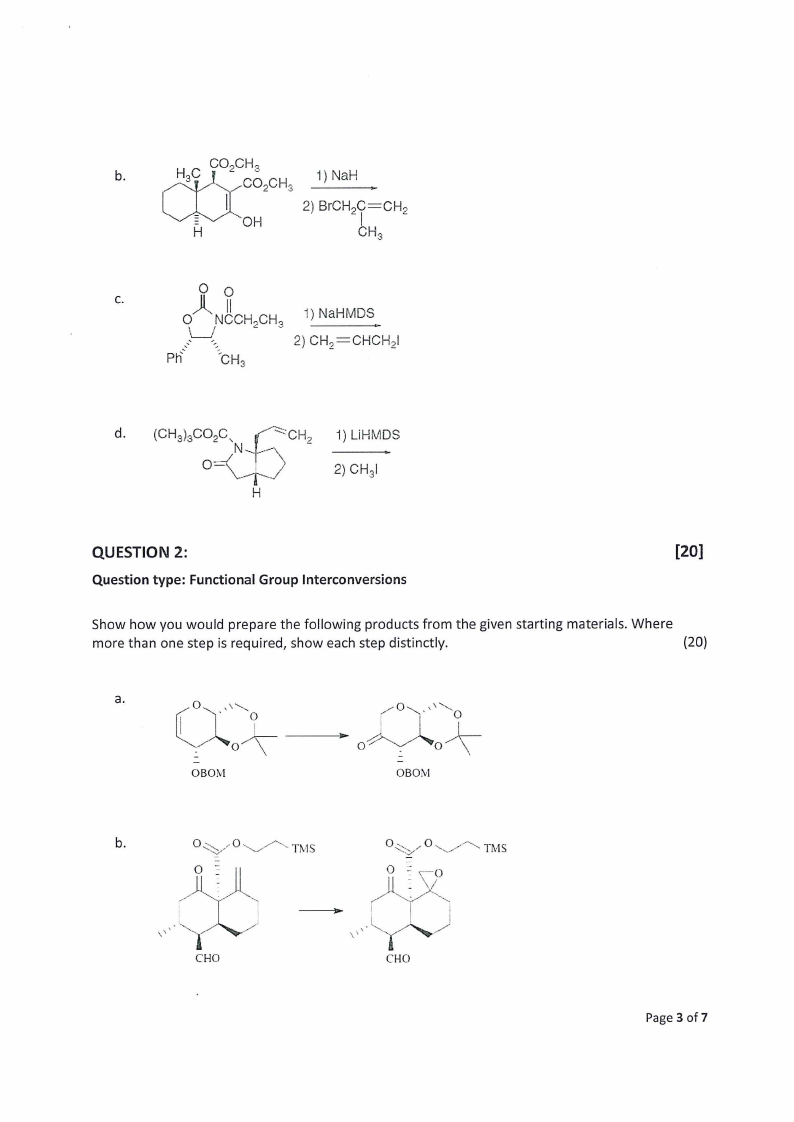
b.
_-CO3 ,CH,
1) NaH
“OH
2) BICH.O=CHp
CH,
Cc
Q i . NCo|OCH,CH,
PR ot CHa
1) NaHMDS
2) CH, =CHCHpl
d. (CHs) BOO.ah ‘CH, 1) LIHMDS
2) CHa
QUESTION 2:
Question type: Functional Group Interconversions
Show how you would prepare the following products from the given starting materials. Where
more than one step is required, show each step distinctly.
a
OOo\\K
2Jk,
AL
OBOM
OBOM
b
Os Os
TMS
Os On ™ TMs
oO c
O =o
CHO
CHO
 |
4 Page 4 |
▲back to top |
QUESTION 3:
[20]
Question type: Protection/Deprotection of functional Groups
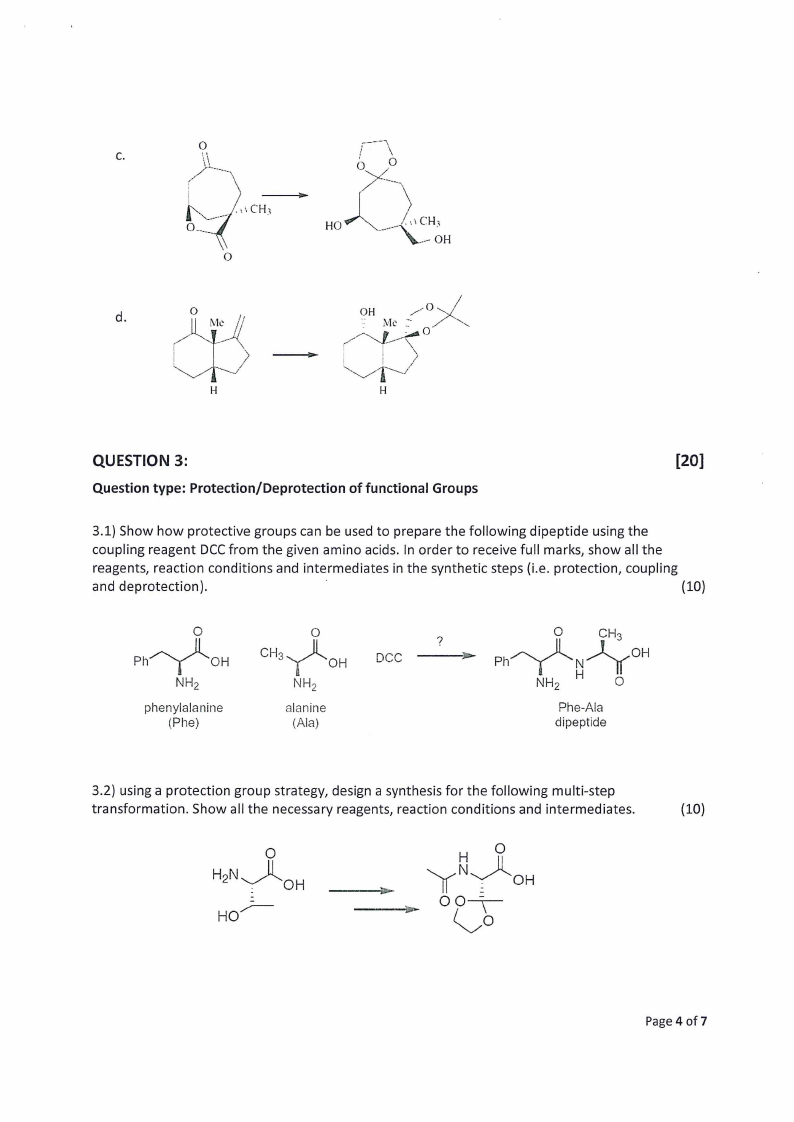
3.1) Show how protective groups can be used to prepare the following dipeptide using the
coupling reagent DCC from the given amino acids. In order to receive full marks, show all the
reagents, reaction conditions and intermediates in the synthetic steps (i.e. protection, coupling
and deprotection).
(10)
oO
NH>
phenylalanine
(Phe)
O
NH,
alanine
(Ala)
9
O
CH3
NH 4 0
Phe-Ala
dipeptide
3.2) using a protection group strategy, design a synthesis for the following multi-step
transformation. Show all the necessary reagents, reaction conditions and intermediates.
(10)
O
HoN Avo
-—
HO
H
O
N or
O O-\\—
O
Page 4 of 7
 |
5 Page 5 |
▲back to top |
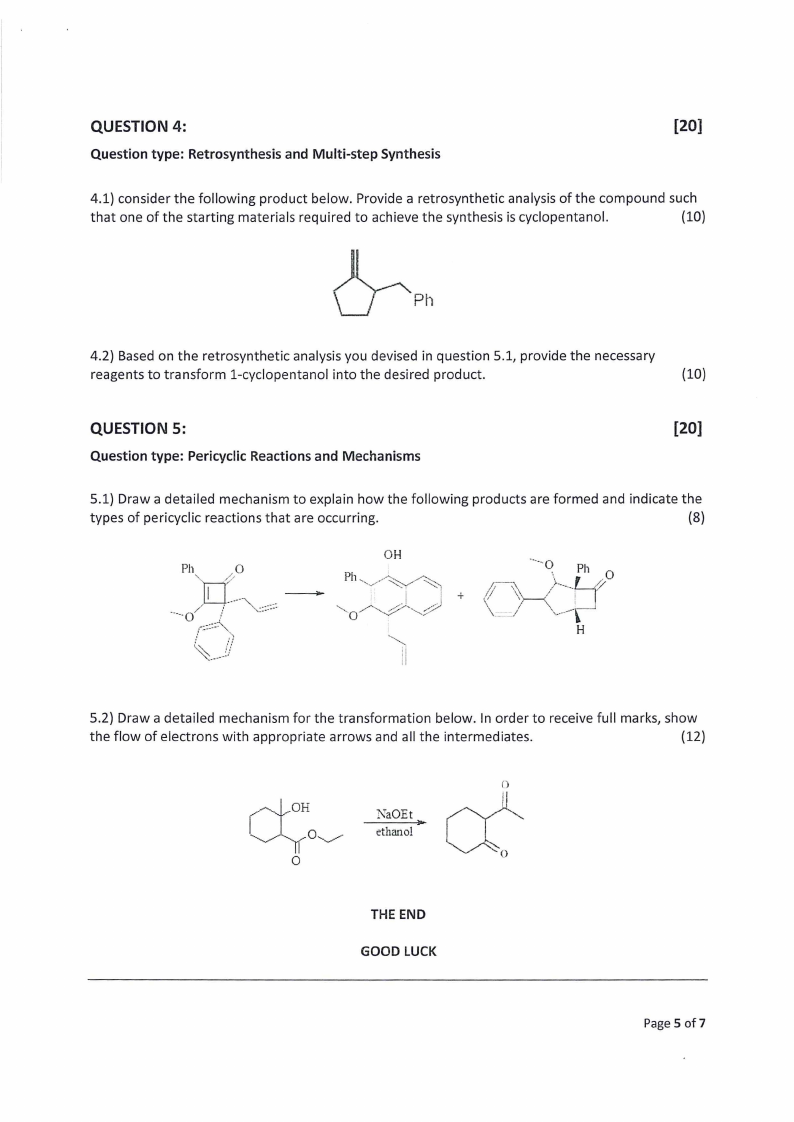
QUESTION 4:
[20]
Question type: Retrosynihesis and Multi-step Synthesis
4.1) consider the following product below. Provide a retrosynthetic analysis of the compound such
that one of the starting materials required to achieve the synthesis is cyclopentanol.
(10)
2
Ph
4.2) Based on the retrosynthetic analysis you devised in question 5.1, provide the necessary
reagents to transform 1-cyclopentanol into the desired product.
(10)
QUESTION 5:
[20]
Question type: Pericyclic Reactions and Mechanisms
5.1) Draw a detailed mechanism to explain how the following products are formed and indicate the
types of pericyclic reactions that are occurring.
(8)
Ph. \\
MyLO
-o ; N aaa
foe.
— i
i
OH
Ph SA i
io
SQN EO| NS , ot
ios
~6
ion
‘Nia Ph zg
v
YX
Keay
wo
H
5.2) Draw a detailed mechanism for the transformation below. In order to receive full marks, show
the flow of electrons with appropriate arrows and all the intermediates.
(12)
QO
OH
NaOEt
{
OL
SeE thanE ol eee
6
O
THE END
GOOD LUCK
Page 5 of 7
 |
6 Page 6 |
▲back to top |
 |
7 Page 7 |
▲back to top |