 |
BPP702S - Biochemistry Biochemical Principles and Practices - 2nd Opp - Jan 2025 |
 |
1 Page 1 |
▲back to top |

nAml BIA UnlVERSITY
OF SCIEnCE AnDTECHnOLOGY
FacultyofHealthN, atural
ResourceasndApplied
Sciences
Schoool f NaturalandApplied
Sciences
Departmentof Biology,
ChemistryandPhysics
QUALIFICATION: BACHELOR OF SCIENCE
QUALIFICATION CODE: 07BOSC
COURSE: BIOCHEMISTRY: BIOCHEMICAL
PRINCIPLES AND PRACTICE
DATE: JANUARY 2025
DURATION: 3 HOURS
13JacksonKaujeuaStreet T: •264 612072012
Private Bag13388
F: •264 612079012
Windhoek
E: dbcp@nust.na
NAMIBIA
W: www.nust.na
LEVEL:7
COURSECODE: BPP702S
SESSION: 1
MARKS: 100
SECOND OPPORTUNITY/ SUPPLEMENTARY: EXAMINATION QUESTION PAPER
EXAMINER:
MODERATOR:
PROF LAMECH MWAPAGHA
DR HARRIS ONYWERA
INSTRUCTIONS:
1. Answer all questions on the separate answer sheet.
2. Please write neatly and legibly.
3. Do not use the left side margin of the exam paper. This must be allowed for the
examiner.
4. No books, notes and other additional aids are allowed.
5. Mark all answers clearly with their respective question numbers.
PERMISSIBLE MATERIALS:
1. Non-Programmable Calculator
This question paper consists of three (3) pages including this front page.
 |
2 Page 2 |
▲back to top |
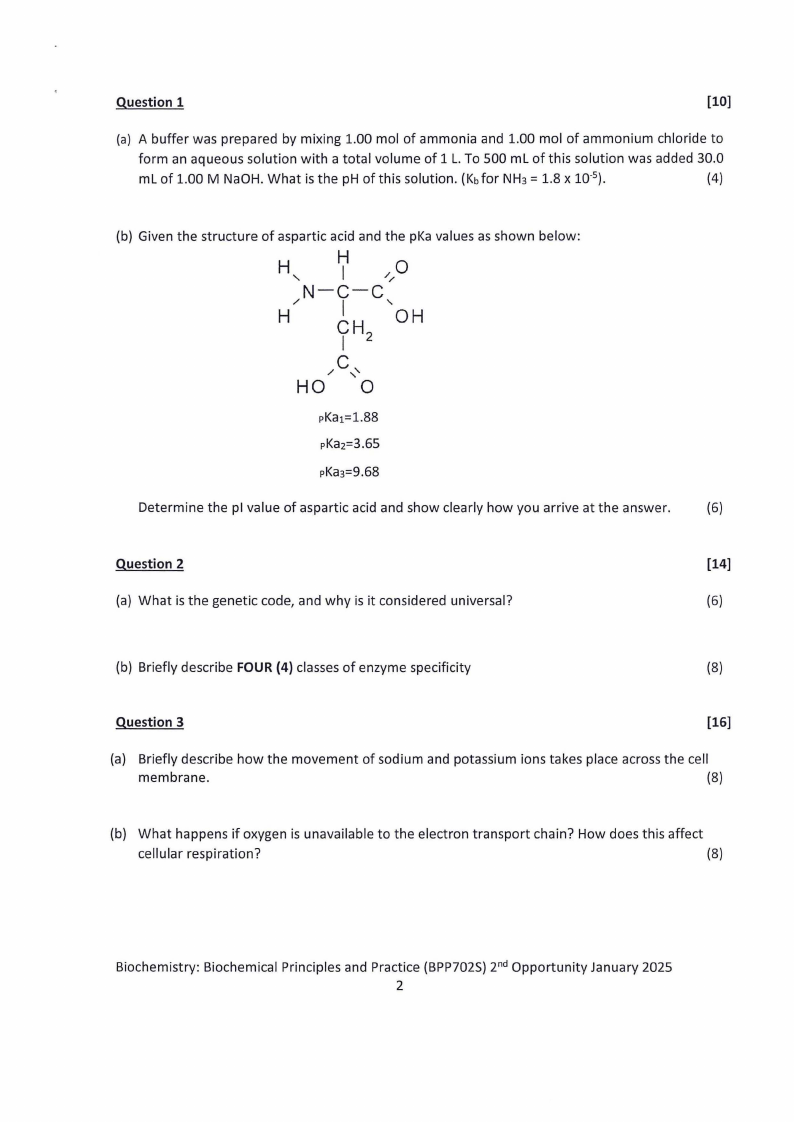
Question 1
[10]
(a) A buffer was prepared by mixing 1.00 mol of ammonia and 1.00 mol of ammonium chloride to
form an aqueous solution with a total volume of 1 L. To 500 ml of this solution was added 30.0
ml of 1.00 M NaOH. What is the pH of this solution. (Kbfor NH3= 1.8 x 10-5).
(4)
(b) Given the structure of aspartic acid and the pKa values as shown below:
H
'
/
HI
N-cI-c
//0
'
H
CH OH
I2
/ C,,
HO 0
pKa1=1.88
pKa2=3.65
Determine the pl value of aspartic acid and show clearly how you arrive at the answer.
(6)
Question 2
[14]
(a) What is the genetic code, and why is it considered universal?
(6)
(b) Briefly describe FOUR (4) classes of enzyme specificity
(8)
Question 3
[16]
(a) Briefly describe how the movement of sodium and potassium ions takes place across the cell
membrane.
(8)
(b) What happens if oxygen is unavailable to the electron transport chain? How does this affect
cellular respiration?
(8)
Biochemistry: Biochemical Principles and Practice (BPP702S)2nd Opportunity January 2025
2
 |
3 Page 3 |
▲back to top |

Question 4
[14]
(a) State SIX (6) functions of the amino acid Threonine
(6)
(b) Briefly discuss the principles of metabolic pathways
(8)
Question 5
[14]
(a) Using structural formulas, write the balanced chemical equation for the reaction where the
production of the electron carrier FADH2takes place in the Kreb cycle.
(6)
(b) With the aid of the fatty acyl CoA structure below, discuss the production of energy
(ATP) through the process of p-oxidation (breakdown) of fatty acids.
(8)
0...
CH3- {CHz)x-CH2- Ct½-C-S-CoA
Fatty acyl CoA
Question 6
[14]
(a) Lipids are known to be insoluble in water, briefly elucidate on how dietary lipid are
digested, absorbed and transported in the body.
(8)
(b) The genetic code is the set of rules defining how the four-letter code of DNA is translated
into amino acids, which are the building blocks of proteins. DiscussTHREE{3} characteristics
of the genetic code
(6)
Question 7
[18]
(a) Discuss FIVE (5) challenges faced during the drug development process and how they
can be addressed.
(10)
(b) Discuss how cholera toxin disrupts the regulation of intestinal secretion following GPCR
signalling.
(8)
THE END
Biochemistry: Biochemical Principles and Practice (BPP702S)2nd Opportunity January 2025
3





