 |
GNC501S - GENERAL CHEMISTRY 1A - 2ND Opp - JULY 2022 |
 |
1 Page 1 |
▲back to top |

o
NAMIBIA UNIVERSITY
OF SCIENCE AND TECHNOLOGY
FACULTY OF HEALTH, APPLIED SCIENCES AND NATURAL RESOURCES
DEPARTMENT OF NATURAL AND APPLIED SCIENCES
QUALIFICATION: BACHELOR OF SCIENCE
QUALIFICATION CODE: 07BOSC
LEVEL: 5
COURSE CODE: GNC501S
COURSE NAME: GENERAL CHEMISTRY 1A
SESSION: JULY 2022
PAPER: THEORY
DURATION: 3 HOURS
MARKS: 100
SUPPLEMENTARY/SECOND OPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S) | DR. EODIA HESS
MODERATOR: | DR. MARIUS MUTORWA
INSTRUCTIONS
Answer ALL the questions.
Write clearly and neatly.
Number the answers clearly
All written work must be done in blue or black ink and sketches can
be done in pencil
|
5. No books, notes and other additional aids are allowed
PERMISSABLE MATERIALS
Non-programmable calculators
ATTACHMENTS
1. List of useful constants
2. Periodic Table
THIS QUESTION PAPER CONSISTS OF 8 PAGES (Including this front page, list of useful constants
and Periodic Table)
 |
2 Page 2 |
▲back to top |

SECTION A: MULTIPLE CHOICE QUESTIONS
[60]
e There are 20 multiple choice questions in this section. Each question carries 3 marks.
e Answer ALL questions by selecting the letter of the correct answer.
e Choose the best possible answer for each question, even if you think there is another
possible answer that is not given.
1. What type of ions have names ending with —ide?
A. Only cations
B. Only anions
C. Only metal ions
D. Only gaseous ions
2. When Group 2A elements form ions, they
A. Lose two protons
B. Lose two electrons
C. Gain two protons
D. Gain two electrons
3. What is the correct name for N37 ion?
A. Nitrate ion
B. Nitride ion
C. Nitrogen ion
D. Nitrite ion
4. Aluminium is a Group 3A metal. Which ion does Al typically form?
A. Al
B. Als+
c. Ale
D. Al>*
5. Bohr’s atomic model ....
A. proposes that electrons occupy specific energy levels.
B. explains the emission spectra of hydrogen atoms.
C . predicts the energy level of multi-electron atoms
D . both A and B
6.
orbitals are spherically symmetrical.
7. Then =1 shell contains
A. 3,6
p sub-orbitals. All other shells contain
Page 2 of 8
p sub-orbitals.
 |
3 Page 3 |
▲back to top |

8. There are
A. 1
orbitals in the second shell.
9. An unknown amount of C3Hg was burned completely to H2O and COz2, with 36 g of H20
recovered.
How many moles of the hydrocarbon were originally present?
A. 0.25
B. 0.50
Cc.
D.
—2386
10. A compound having an empirical formula of SO3 is found to have a molecular weight of 80.
What is its molecular formula?
A. S309
B. S206
C. SO3
D. SOg
11. Balance the following reaction:
S$ +02 —- SO3
S + 03 > SO3
4S + 202 > 4803
2S + 302 > 2S03
3S + 202 > 3S03
12. What is the formula weight of Al2(SOa)3?
A. 150
B. 123
C. 342
D. 315
13. Standard conditions (STP) are:
0°C and 2 atm
32°F and 76 torr
273 K and 760 mmHg
4°C and 7.6 mmHg
Page 3 of 8
 |
4 Page 4 |
▲back to top |
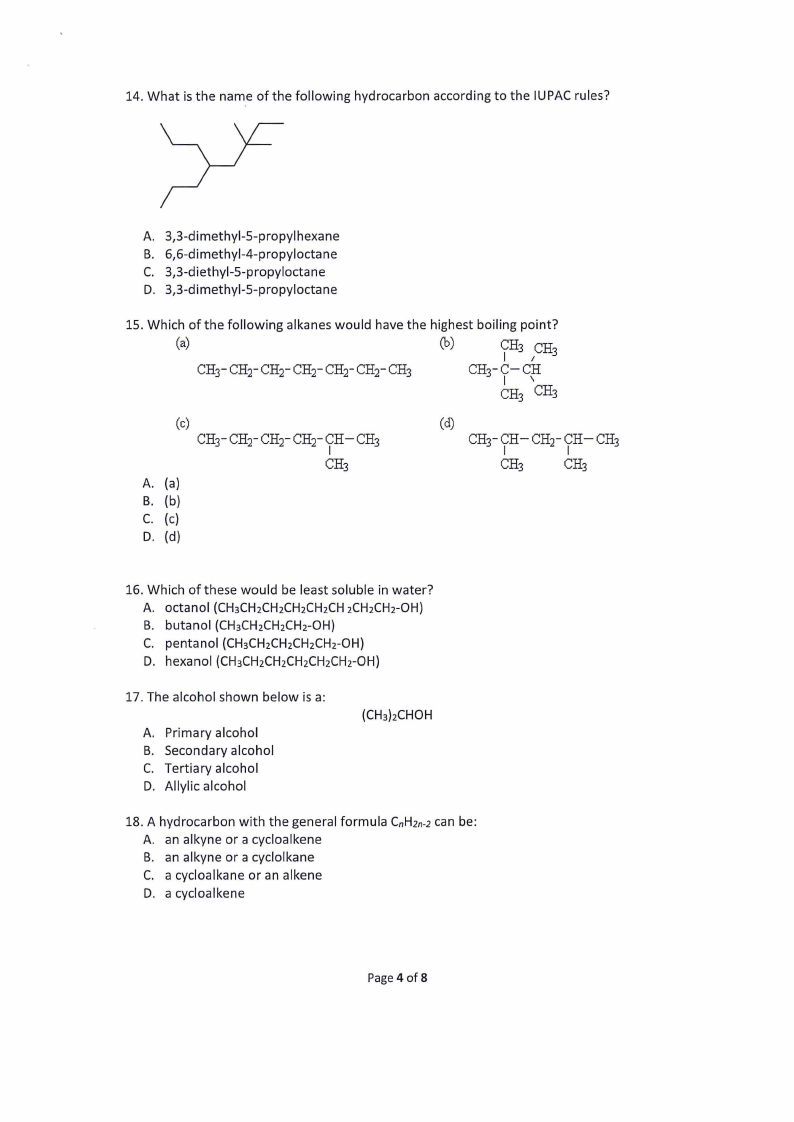
14. What is the name of the following hydrocarbon according to the IUPAC rules?
3,3-dimethyl-5-propylhexane
6,6-dimethyl-4-propyloctane
3,3-diethyl-5-propyloctane
3,3-dimethyl-5-propyloctane
15. Which of the following alkanes would have the highest boiling point?
(a)
(b)
ve (CHs
CH3- CH)- CH)- CH)- CH)- CH)- CH3
CH3- Co CH
CH, CH3
(c) CH3- CH)- CH” CHy- CH— CH;
(d)
CH3- CH CH” CH CH3
CH3
(a)
(b)
(c)
(d)
CH;
CH;
16. Which of these would be least soluble in water?
octanol (CH3CH2CH2CH2CH2CH 2CH2CH2-OH)
butanol (CH3CH2CH2CH2-OH)
pentanol (CH3CH2CH2CH2CH2-Oh)
hexanol (CH3CH2CH2CH2CH2CH2-OH)
17. The alcohol shown below is a:
Primary alcohol
Secondary alcohol
Tertiary alcohol
Allylic alcohol
(CH3)2CHOH
18. A hydrocarbon with the general formula C,H2n-2 can be:
A. an alkyne or a cycloalkene
B. an alkyne or a cyclolkane
C. a cycloalkane or an alkene
D. acycloalkene
Page 4 of 8
 |
5 Page 5 |
▲back to top |

19. Smoke is an example
A. afoam
B. an aerosol
Cc. agel
D. sol
of a colloid
termed:
20. What is the boiling point in °C of a solution of a 2.15 m aqueous solution of glycerol? (Kp =
0.512°C/m)?
A. 101.1
B. 100.2
c. 100
D. 1.1
SECTION B:
[40]
There are FIVE questions in this section. Answer all Questions.
Show clearly, where necessary, how you arrive at the answer as the working will carry marks to.
Question 1
[10]
a) All alkali metals react with water to produce hydrogen gas and the corresponding alkali metal
hydroxide. A typical reaction is that between lithium and water:
Li (s) + H20 (I) > LiOH (aq) + H2 (g)
How many grams of Li is needed to produce 9.89 g of H2?
(2)
b) Titanium is prepared by the reaction of titanium(IV) chloride with molten
950°C and 1150°C.
TiCla (g) + Mg (I) > Ti (s) + MgCle (I)
If 3.54 x 107g of TiClq reacts with 1.13 x 10” g of Mg:
(i) Calculate the theoretical yield of Tiin grams.
(ii) Calculate the percent yield if 7.91 x 10° g of Ti are actually obtained.
magnesium
between
(6)
(2)
Question 2
[6]
a) How many grams of potassium dichromate are required to prepare a 250 mL solution
whose concentration is 2.16 M?
(3)
b) Describe how you would prepare 5.00 x 10? mL of a 1.75 M sulphuric acid solution, starting
with a 8.16 M stock solution.
(3)
Question 3
[8]
a) Sulfur hexafluoride is a colorless and odourless gas. Due to its lack of chemical reactivity, it is
used as an insulator in electronic equipment. Calculate the pressure (in atm) exerted by 1.82
moles of gas in a steel vessel of volume 5.43 Lat 69.5 °C.
(2)
Page 5 of 8
 |
6 Page 6 |
▲back to top |
b) Calculate the volume (in L) occupied by 7.40 g of ammonia at STP.
(2)
c) A flammable gas made up of carbon and hydrogen is found to effuse through a porous barrier
in 1.50 min. Under the same conditions and pressure it takes an equal volume of bromine
vapour 4.73 min to effuse through the same barrier. Calculate the molar mass of the unknown
gas.
(4)
Question 4
[12]
a) Calculate the pH of (i) a 1.0 x 10° M HCI solution and (ii) 0.020 M Ba(OH), solution.
(6)
b) Calculate the pH of a 0.036 M nitrous acid (HNOz2) solution:
(6)
HNO> (aq) @ H+ (aq) + NOx (aq)
Question 5
[4]
a) Write the IUPAC names of the following compounds:
(2)
(i)
NS
(ii)
(b) State the functional group in each of the following compounds:
(3)
|
(ii)
CH; ——Cc ——OH
END OF EXAMINATION
Page 6 of 8
 |
7 Page 7 |
▲back to top |

USEFUL CONSTANTS:
Gas constant, R = 8.3145J- mol: K?= 0.083145 dm?: bar: mol: K+= 0.08206 L atm mol?: K?
1Pa:m?=1kPa.l =1N-m =14J
1 atm = 101 325 Pa = 760 mmHg = 760 torr
Avogadro’s Number, Na = 6.022 x 1023 mol
Planck’s constant, h = 6.626 x 1074 Js
Speed of light, c = 2.998 x 108 ms?
Page 7 of 8
 |
8 Page 8 |
▲back to top |
Page 8 of 8





