 |
QPH702S- QUANTUM PHYSICS - JAN 2020 |
 |
1 Page 1 |
▲back to top |

NAMIBIA UNIVERSITY
OF SCIENCE AND TECHNOLOGY
FACULTY OF HEALTH AND APPLIED SCIENCES
DEPARTMENT OF NATURAL AND APPLIED SCIENCES
QUALIFICATION : BACHELOR OF SCIENCE
QUALIFICATION CODE: 07BOSC
LEVEL: 7
COURSE NAME: QUANTUM PHYSICS COURSE CODE: QPH702S
SESSION: JANUARY 2020
DURATION: 3 HOURS
PAPER: THEORY
MARKS: 100
SUPPLEMENTARY/SECOND OPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S) | Prof Dipti R. Sahu
MODERATOR: | Dr Habatwa V. Mweene
INSTRUCTIONS
1. Answer any five questions.
2. Write clearly and neatly.
3. Number the answers clearly.
PERMISSIBLE MATERIALS
Non-programmable Calculators
THIS QUESTION PAPER CONSISTS OF 4 PAGES (Including this front page)
 |
2 Page 2 |
▲back to top |
Question 1
[20]
The wave function ofa particle moving in the x-dimension is
(x) = Nx(L—x) O<x<L
0
elsewhere
(a) Normalize the wave function
(5)
(b) Determine the expectation value of x
(5)
(c) Calculate <p,>, <p,2> and Apx
(10)
Question 2
[20]
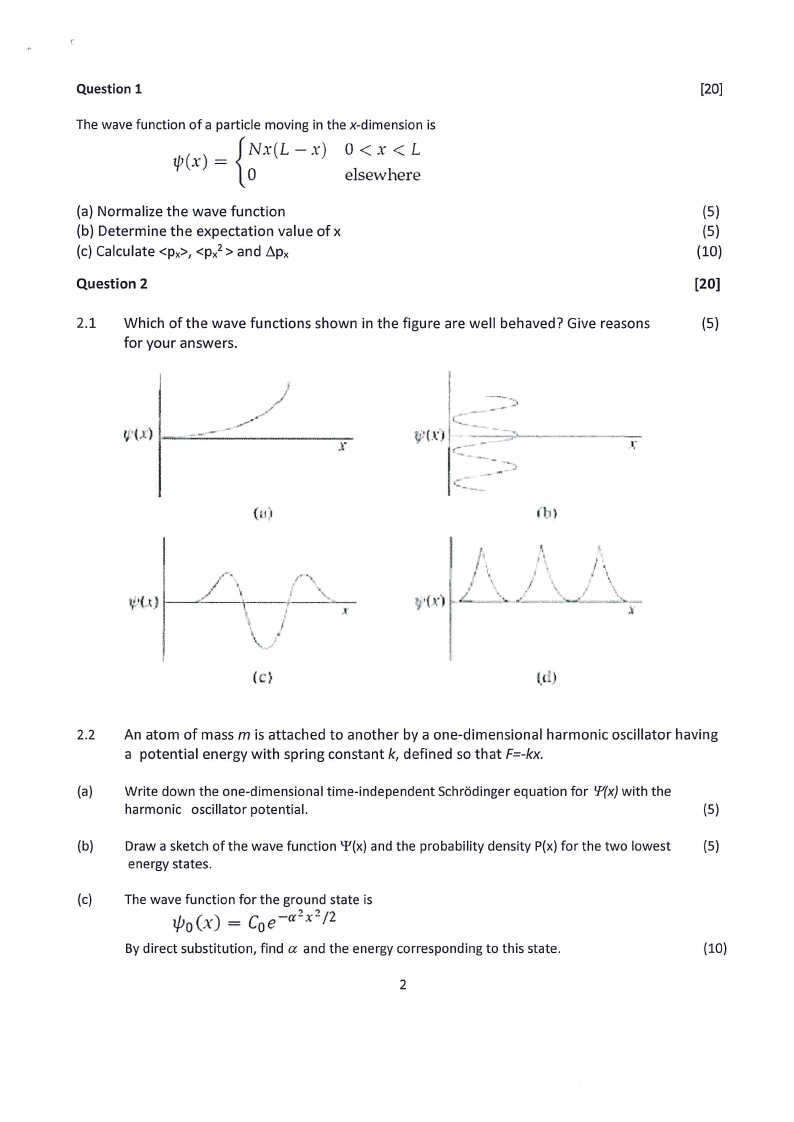
2.1 Which of the wave functions shown in the figure are well behaved? Give reasons
(5)
for your answers.
|
|
yx)
aa
¥
yey | : seaen
——'
ii
th}
f
*
J Ne
wtr)
}~
\\
(c}
|i
fs
‘
yx | ne
|
Fi
/ cs
}
™
“y
ce
2.2 An atom of mass m is attached to another by a one-dimensional harmonic oscillator having
a potential energy with spring constant k, defined so that F=-kx.
(a)
Write down the one-dimensional time-independent Schrédinger equation for ¥/(x) with the
harmonic oscillator potential.
(5)
(b)
Draw a sketch of the wave function ‘¥(x) and the probability density P(x) for the two lowest
(5)
energy states.
(c)
The wave function for the ground state is
Wo (x) = Co eax? /2
By direct substitution, find @ and the energy corresponding to this state.
(10)
2
 |
3 Page 3 |
▲back to top |

Question 3
[20]
3.1
An electron has a kinetic energy of 12.0 eV. The electron is incident upon a rectangular barrier
of height 20.0 eV and thickness 1.00 nm. By what factor would the electron’s probability of
tunneling through the barrier increase assuming that the electron absorbs all the energy of a
photon with wavelength 546 nm (green light)?
(5)
3.2 The potential function V(x) of the problem is given by
V, x >0
V(x) =
0 x< 0
where Vo is a constant potential energy.
(a) Sketch the graph of this function
(2)
(b) Find the wave function for ¢ <V. where ¢ is the incident particle energy and
interpret the results.
(13)
Question 4
[20]
4.1
What are the kinetic, potential and Hamiltonian operators for the hydrogen atom? Write the
Schrodinger equation for the H-atom.
(5)
4.2
Show, for Hermitian operators A and B, that the product AB is a Hermitian operator if
and only ifA and B commute.
(5)
4.3
Show explicitly in Cartesian coordinates(x, y, z) that the operators V? and L, commute, i.e.,
[v* , f]=0.
(10)
Question 5
[20]
5.1 What are the Pauli spin matrices and to what value of spin they correspond? Write
them down.
(5)
Sad For each Pauli matrix, find its eigenvalues, and the components of its normalized
eigenvectors in the basis of the eigenstates of S:.
(10)
5.3 Evaluate the matrix of Ly for / = 1. Why is the matrix not diagonal?
(5)
 |
4 Page 4 |
▲back to top |
Question 6
[20]
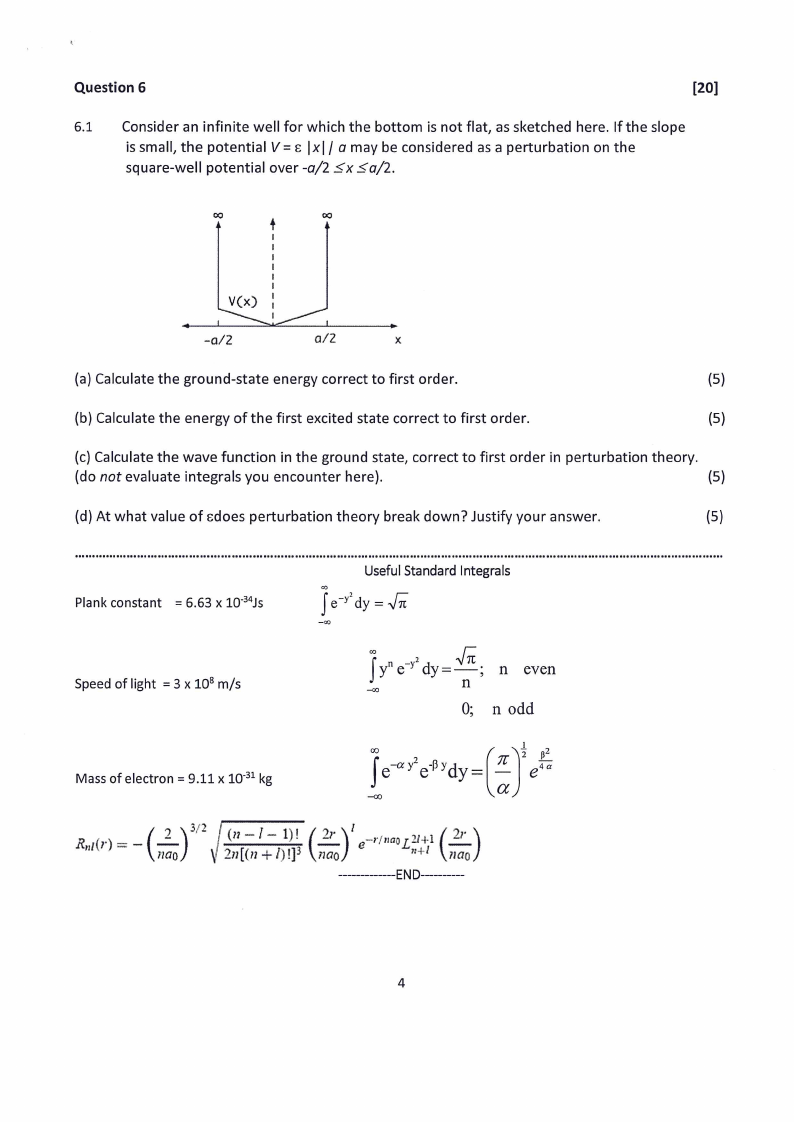
6.1 Consider an infinite well for which the bottom is not flat, as sketched here. If the slope
is small, the potential V = |x|/ a may be considered as a perturbation on the
square-well potential over -a/2 <x <a/2.
<<
j
-a/2
1
>
a/2
x
(a) Calculate the ground-state energy correct to first order.
(5)
(b) Calculate the energy of the first excited state correct to first order.
(5)
(c) Calculate the wave function in the ground state, correct to first order in perturbation theory.
(do not evaluate integrals you encounter here).
(5)
(d) At what value of edoes perturbation theory break down? Justify your answer.
(5)
Plank constant = 6.63 x 10°4Js
Speed of light = 3 x 10® m/s
Mass of electron = 9.11 x 1037 kg
Useful Standard Integrals
ao
feway = Vn
-oO
Tlyen e* y? dy=V—r;.
on
n
0;
n even
n odd
0
x+
ay” 2 Byq,,
|
+4a
fe e"’dy= (=| e€
00
Ry) = — (2.)"
“ee
nag
\\) (2un—[I~(+ QDIrItP
(2)
\\nao
—rjnag
e
7
U4]
nl
\\(j=ag)
sree —END———-





