 |
CLC611S - CLINICAL CHEMISTRY 2A - 1ST OPP - JULY 2022 |
 |
1 Page 1 |
▲back to top |
nAmlBIA unlVERSITY
OF SCIEnCE Ano TECHnOLOGY
FACULTYOF HEALTH,APPLIEDSCIENCESAND NATURALRESOURCES
DEPARTMENT OF HEALTH SCIENCES
QUALIFICATION: BACHELOR OF MEDICAL LABORATORY SCIENCES
QUALIFICATION CODE: 08BMLS
LEVEL: 6
COURSE CODE: CLC611S
COURSE NAME: CLINICAL CHEMISTRY 2A
SESSION:
JUNE 2022
PAPER:
THEORY
DURATION:
3 HOURS
MARKS:
100
FIRSTOPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S)
Dr Elzabe van der Coif
MODERATOR:
Dr Maurice Nyambuya
INSTRUCTIONS
1. Answer ALL the questions.
2. Write clearly and neatly.
3. Number the answers clearly.
PERMISSIBLEMATERIALS
1. Calculator
THIS QUESTION PAPER CONSISTS OF 6 PAGES {Including this front page)
 |
2 Page 2 |
▲back to top |

QUESTION 1
[7]
Select the ONE best answer from the options provided. One mark for each correct
answer.
1.1 The following is not true of arterial blood:
A. Most commonly used for blood gas analysis
B. Is uniform throughout the body
C. Should only be collected by a doctor
D. The pH, pC02 and p02 reflect respiratory and metabolic status
1.2 The following in not true of venous blood:
A. The content varies throughout the body
B. Based on the metabolic activity of the surrounding tissue
C. Content varies by collection site and/or collection time
D. Should only be collected early morning
1.3 The following analytes will differ between venous and arterial blood:
A. 02, pH, CO2
B. Glucose
C. Packed cell volume
D. Chloride and ammonia
E. All of the above
1.4 When not to use skin puncture and collect capillary blood:
A. When patient has good veins for collection of venous blood
8. Venous sites limited/not accessible, eg. burn wounds
C. Fragile veins, eg. elderly patients
D. Risk factors, eg. infants and neonates
1.5 The following physiological factors affect the specimen, except:
A. Time of collection
B. Food intake
C. Insomnia
D. Exercise
E. Emotional stress
2
 |
3 Page 3 |
▲back to top |

1.6 Pre-analytical errors include the following, except:
A. Patient-related aspects eg. posture
B. Controls which are out of range
C. Collection-related aspects eg. haemolysis
D. Processing-related aspects eg. clotting time
E. Storage-related aspects eg. wrong temperature
1.7 Analytical sensitivity of a test method is the ability of the test to:
A. Only test positive for the analyte being investigated
B. Report true positive results
C. Predict the positive results in patients who have the disease
D. Predict the negative results in patients who do not have the disease
QUESTION 2
[7]
Indicate whether the following statements are TRUE or FALSE.One mark for each
correct answer.
2.1 Most errors in the clinical laboratory are in the analytical phase.
2.2 The laboratory is not responsible for the preparation of the patient and the
correct collection of blood samples for analysis.
2.3 When a phlebotomist collects a blood sample, she must first write the name of
the patient on the Vacutainer before collecting the sample.
2.4 All patient samples handled in the laboratory should be treated as if they are
infectious.
2.5 It takes 20-30 minutes for a clot to form at room temperature, unless a clot
activator is used.
2.6 If serum is separated too quickly, micro-clots and fibrinogen may be present.
2.7 If serum is not removed in a timely fashion, WBC and RBCwill begin to metabolise
glucose in the serum.
3
 |
4 Page 4 |
▲back to top |

QUESTION 3
[21]
3.1 Identify the basic SI units of weight, volume and length.
(3)
3.2 Name three ways how the concentration of a solution can be expressed. (3x2=6) (6)
3.3 Define each of the three ways mentioned in 3.2. (3x2=6}
(6)
3.4 Give an example of each of the three ways mentioned in 3.2. (3x2=6)
(6)
QUESTION 4
[15]
4.1 Physiological saline is 0.15 M of NaCl. The molecular weight of NaCl is 58.44
g/mol. Show your calculations and determine how much NaCl needs to be
weighed out to make 500 ml of physiological saline. Round off your answer to
two decimals.
(5)
4.2 Explain the steps which you would follow to make up 1 litre of 1 Molar of
hydrochloric acid. The molecular weight of HCI is 36.46 g/mol.
(10)
QUESTION 5
[15]
Your laboratory bought a commercial control for cholesterol from the manufacturer.
The package insert provides the following values for the mean and range (including
two standard deviations from the mean on each side):
Mean: 4.03 mmol/I
Range: 3.22 - 4.83 mmol/I
5.1 Calculate the standard deviations which you would use in a Levey-Jennings chart
to plot the control values for a cholesterol test.
(5)
5.1.1 Two standard deviations below the mean
5.1.2 One standard deviation below the mean
5.1.3 The mean
5.1.4 One standard deviation above the mean
5.1.5 Two standard deviations above the mean
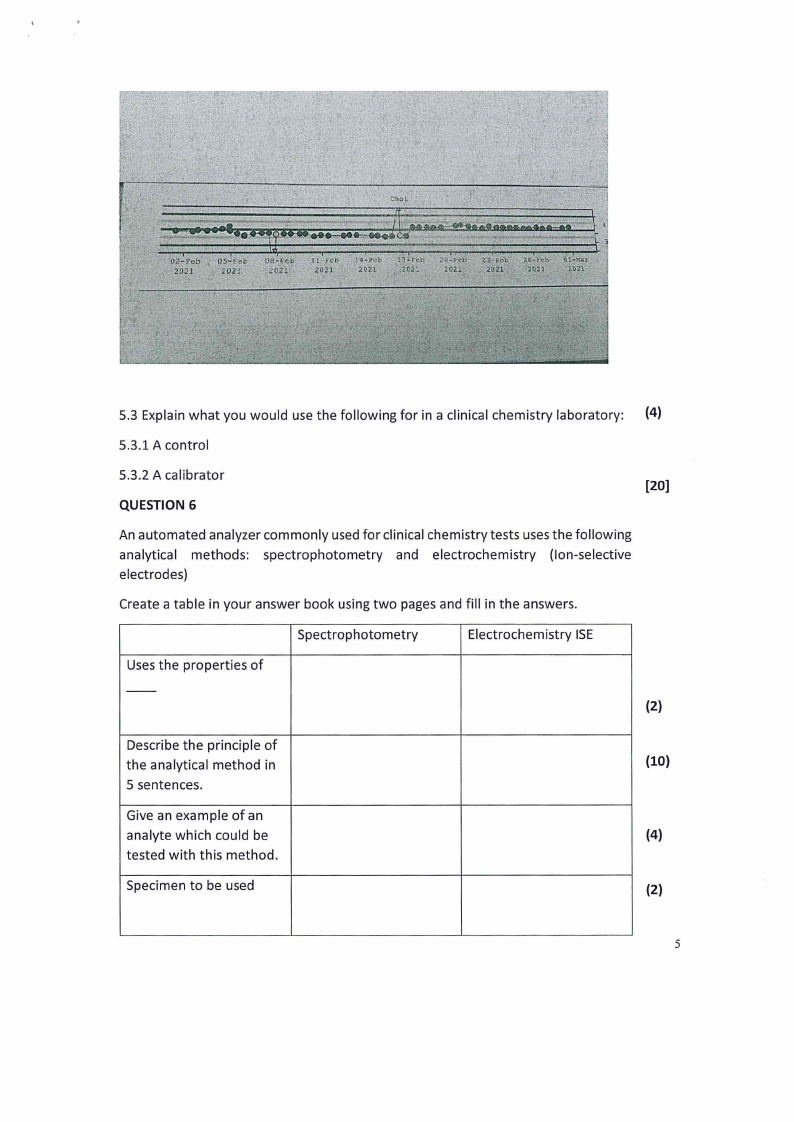
5.2 Evaluate the following Levey-Jennings chart for cholesterol and indicate which
Westgard rules have been violated on which dates. (3x2=6)
An arrow means the value exceeded three standard deviations from the mean.
(6)
4
 |
5 Page 5 |
▲back to top |
5.3 Explain what you would use the following for in a clinical chemistry laboratory: (4)
5.3.1 A control
5.3.2 A calibrator
[20]
QUESTION 6
An automated analyzer commonly used for clinical chemistry tests uses the following
analytical methods: spectrophotometry and electrochemistry (Ion-selective
electrodes)
Create a table in your answer book using two pages and fill in the answers.
Spectrophotometry
Electrochemistry ISE
Uses the properties of
--
{2)
Describe the principle of
the analytical method in
{10)
5 sentences.
Give an example of an
analyte which could be
(4)
tested with this method.
Specimen to be used
(2)
5
 |
6 Page 6 |
▲back to top |

Influenced by the
presence of
(2)
haemolysis/bilirubin Y/N
[10]
QUESTION 7
Calculate the answers to the following problems related to dilutions.
7.1 One ml serum is mixed with 9 ml diluent.
What is the resulting dilution?
What is the dilution factor?
(3)
7.2 A 1 to 2 dilution of serum is needed.
The total volume must equal 100 µL.
What volume of serum and diluent are needed?
(3)
7.3 A patient's cholesterol result is outside the linear range of the analyzer.
10 µL of serum is added to 190 µL of diluent, and the diluted sample is
re-analyzed. The value of the diluted sample is 0.70 mmol/1.
What cholesterol value should be reported to the doctor?
(4)
QUESTION 8
[S]
Motivate why you think point of care testing is valuable in the health sector and
explain how you see the role of the Medical Laboratory Scientist in point of care
testing.
6





