 |
PNM710S - Pyrometallurgy of Non-Ferrous Metals - 1st Opp - June 2022 |
 |
1 Page 1 |
▲back to top |

 |
2 Page 2 |
▲back to top |

 |
3 Page 3 |
▲back to top |

 |
4 Page 4 |
▲back to top |

 |
5 Page 5 |
▲back to top |

 |
6 Page 6 |
▲back to top |

 |
7 Page 7 |
▲back to top |
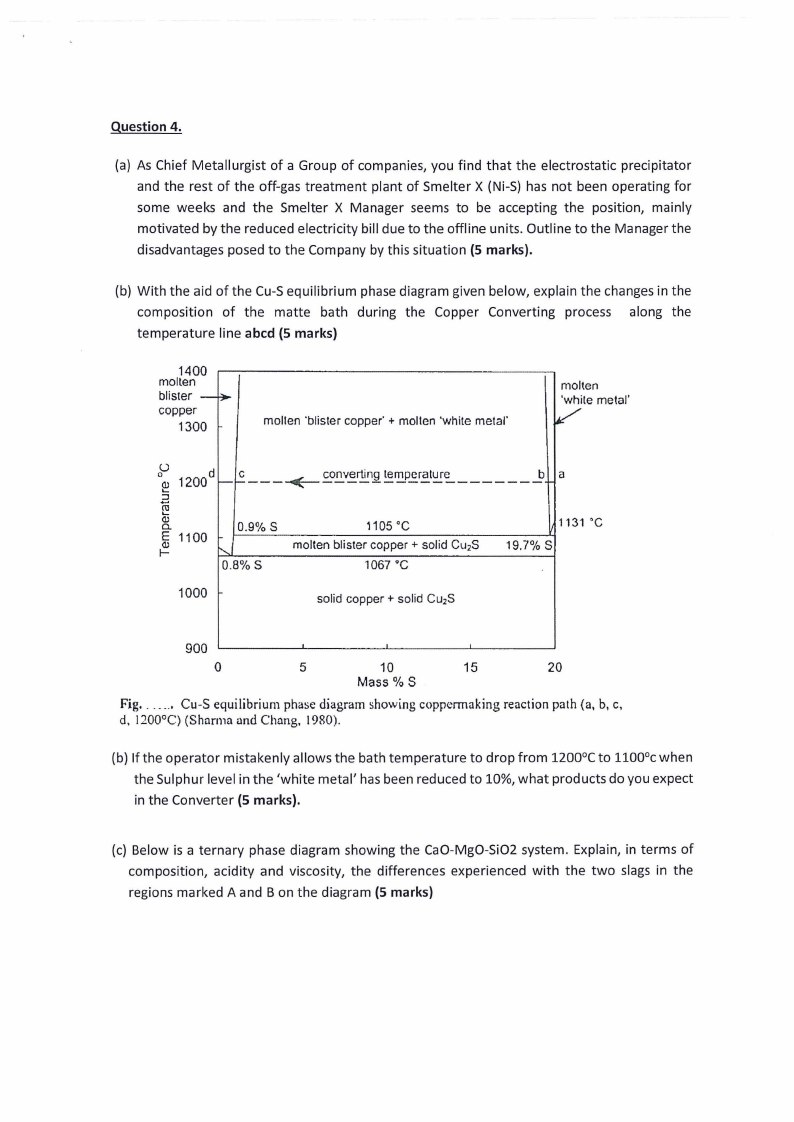
 |
8 Page 8 |
▲back to top |

 |
9 Page 9 |
▲back to top |
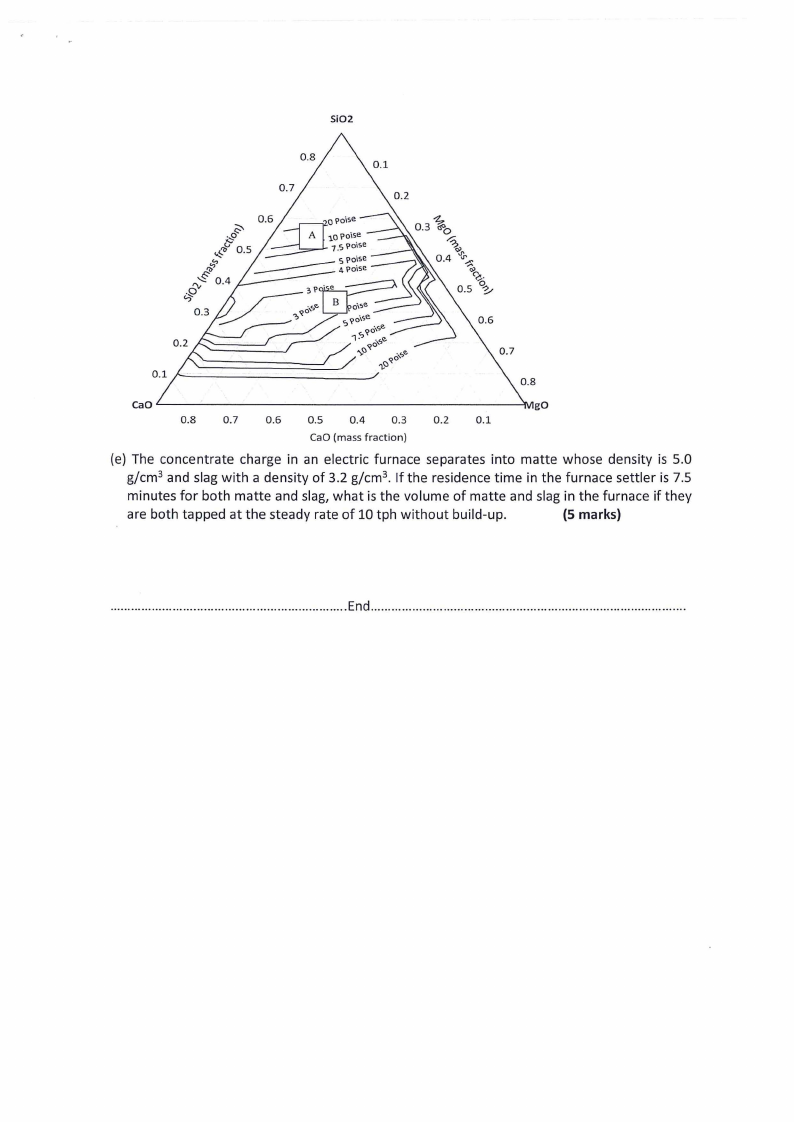
 |
10 Page 10 |
▲back to top |






