 |
CEN810S - Corrosion Engineering 414 - 2nd OPP - JUN 2023 |
 |
1 Page 1 |
▲back to top |

n Am I B I A u n IVER s I TY
OF SCIEnCE Ano TECHnOLOGY
FACULTY OF ENGINEERING AND THE BUILT ENVIRONMENT
DEPARTMENT OF CIVIL, MINING AND PROCESSENGINEERING
QUALIFICATION: BACHELOR OF ENGINEERING IN METALLURGY
QUALIFICATION CODE: 08BMET
LEVEL: 8
COURSECODE: CEN810S
COURSE NAME: Corrosion Engineering 414
SESSION:June 2023
DURATION: 2 HOURS
PAPER:2
MARKS: 70
EXAMINER($)
SECOND OPPORTUNITY PAPER
Prof D Groot
MODERATOR:
Prof J van der Merwe, University of the Witwatersrand
INSTRUCTIONS
1. Answer all questions.
2. Read all the questions carefully before answering.
3. Marks for each question are indicated at the end of each question.
4. Please ensure that your writing is legible, neat and presentable.
PERMISSIBLE MATERIALS
1. Examination paper.
2. Scientific calculator, non-programmable
THIS PAPER CONSISTS OF 4 PAGES (including this front page)
 |
2 Page 2 |
▲back to top |

Question 1
Passivation plays an important role in corrosion of metals and alloys.
(a) Describe the behaviour of an "active-passive" metal when a fresh surface is exposed to a corrosive
environment. Draw a schematic Evans diagram to illustrate your discussion, labelling the various regions
on the diagram. Indicate important potentials and current densities.
[10]
(b) Give an example of such a metal or alloy.
[1]
Question 2
The formation of mill scale during certain processing of e.g. steel profiles is considered a form of
corrosion.
(a) Compare this type of corrosion to the other broad class of corrosion mechanisms, and discuss
how this type may be modeled.
[7]
(b) Discuss the effect of metal oxide volume on the protective ability of an oxide layer on a metal.
[8]
Question 3
lntergranular corrosion {IGC) is a localised corrosion that occurs at or near the grain boundaries in a metal or
alloy.
(a) State at least three general factors that can cause this type of corrosion.
[3]
(b) State what the main preventative measures against IGC are that you can take during the metal alloying
stage.
[2]
Question 4
Consider an unpainted mild steel item exposed to the atmosphere. After some time, examination of the
piece shows general corrosion. Hint: keep in mind the practical experiment you did.
Explain, in terms of the basic aqueous corrosion cell, how this type of corrosion has occurred.
[9]
Question 5
A brass (copper-based alloy) fitting used in a marine application is joined by soldering with lead-tin solder. This
application is used even in fresh water plumbing systems.
(a) Do you expect corrosion to occur? Explain your answer.
[3]
{b) Explain the advantages and disadvantages of this type of joining.
[2]
Question 6
Cathodic protection may be achieved by the use of an impressed current from an electrical source, or by the
use of sacrificial anodes. Discuss the merits and demerits of sacrificial anodes approach.
[6)
Question 7
Consider a pipeline made of high strength steel, carrying a flow of seawater to a desalination plant. One part
is encased in concrete, running into the sea. The rest is buried in moist soil, running to the plant. At the plant
it is connected via bolted flanges to an electrical pump. The pump housing is a casting in naval brass.
(a) Is stress corrosion cracking likely, in your opinion? Explain your answer.
[3]
(b) What (other) type(s) of corrosion may be expected in this situation? What could be done to manage the
problem(s)?
[9]
2
 |
3 Page 3 |
▲back to top |
Question 8
Consider a concentration cell made up of two Zn electrodes. One is immersed in a deaerated ZnS04 solution
at 0.2 mol/1, and the other in a deaerated ZnS04 solution at 0.6 mol/1.
(a) Calculate the cell potential.
[3]
(b) State which electrode is the anode and explain why.
[2]
(c) State your assumptions.
[2]
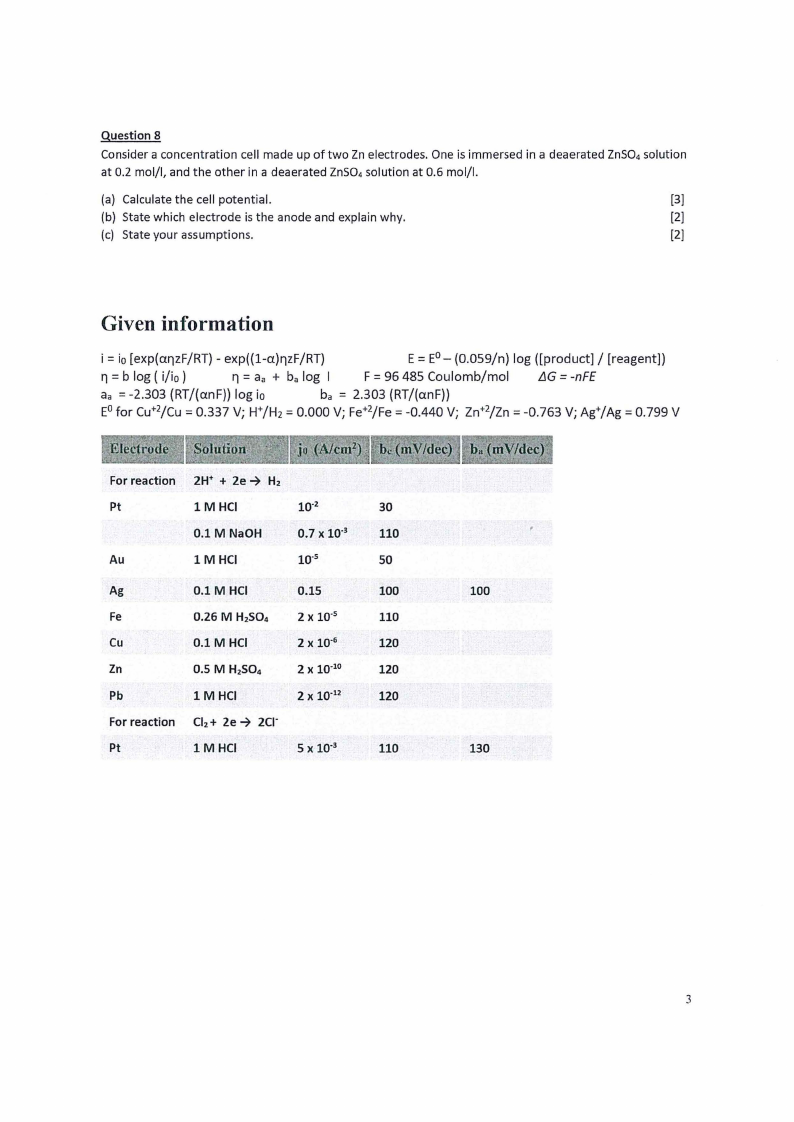
Given information
i = io[exp(ar,zF/RT) - exp((l-a)r,zF/RT)
E = E0 - (0.059/n) log ([product]/ [reagent])
ri =b log ( i/io)
ri =aa + ba log I
F =96 485 Coulomb/mo!
LlG = -nFE
= aa -2.303 (RT/(anF)) log io
= ba 2.303 (RT/(anF))
= = = = E0 for cu+2/Cu 0.337 V; W/H 2 0.000 V; Fe+2/Fe -0.440 V; zn+2/Zn -0.763 V; Ag+/Ag = 0.799 V
For reaction 2W +
Hz
Pt
lM HCI
10-2
30
0.1 M NaOH
0.7 X 10'3
110
Au
1 M HCI
10·5
50
Ag
0.1 M HCI
0.15
100
100
Fe
0.26 M H2S04 2 X 10·5
110
Cu
0.1 M HCI
2xl0· 6
120
Zn
0.5 M H2S04
2 X 10"10
120
Pb
1 MHCI
2 X 10"12
120
For reaction Clz+ 2e 2c1·
Pt
lMHCI
5 X 10"3
110
130
3
 |
4 Page 4 |
▲back to top |
Galvanic series in seawater
0.2 0 -0.2 -0.4 -0.6 -0.8 -1.0 -1.2 -1.4 -1.6
III
Magn('sium
I Zinc
BcQ'llium
.. I Aluminium ullovs
I Cadmium
·
l Mild steel & Cast iron
- I Low ulloysteel
Austenitic rnst iron
Aluminium bronze
Narnl brass,yl'llow brass & l'<'d brass
Tin
Copp'-'r
50/50 lead tin sold<•r
Admiralty brass, nlurniniurn bmss
Manganese bronze
Silicon bronze
Stainless st('el - grades -H0, -tl 6
Nickel sih·er
90/10 coppt'r nickel
80/20 copper nickel
Stainless steel - gradr -430
Lead
70/30 copper nickel
Nickel aluminium bronze
Nickel <:hromium alloy 600
Nickel 200
Silver
Stainless steel - grades 302, 304, 321 & 3-47
Nickel copper allo)S - 400, K500
9
Stainless steel• grades 316 & 317
Alloy 20 sb1inlcss st<'el
Nickel iron chromium alloy 825
Titanium
j
I I Gold, platinum
Gr;1phitc
MOST NOBLE- CATHODIC
'I
!I
LEAST NOBLE - ANODIC
http://ww\\v.ssina.com/i1nagcsicorrosion!ga)vanic-scrics.gif
4





