 |
HMT710S - Hydrometallurgy 314 - 2nd Opp - June 2023 |
 |
1 Page 1 |
▲back to top |

n Am I BI A u n IVE Rs ITY
OF SCIEn CE Ano TECHn OLOGY
FACULTY OF ENGINEERING AND SPATIAL SCIENCES
DEPARTMENT OF MINING AND PROCESS ENGNEERING
QUALIFICATION : BACHELOR OF ENGINEERING IN METALLURGY
QUALIFICATION CODE: 08BEMT
LEVEL: 7
COURSE CODE: HMT 710S
COURSE NAME: HYDROMETALLURGY 314
SESSION: JUNE 2023
DURATION: 2.5 HOURS
PAPER: THEORY
MARKS: 75
SECOND OPPORTUNITY QUESTION PAPER
EXAMINER(S} Mr. Bernard Sililo
Ms Foibe Uahengo
MODERATOR: Dr. Theresa Coetsee
INSTRUCTIONS
1. Answer all questions.
2. Read all the questions carefully before answering.
3. Marks for each question are indicated at the end of each question.
4. Please ensure that your writing is legible, neat and presentable.
PERMISSIBLE MATERIALS
1. Examination paper.
THIS QUESTION PAPER CONSISTS OF 10 PAGES {Including this front page)
 |
2 Page 2 |
▲back to top |
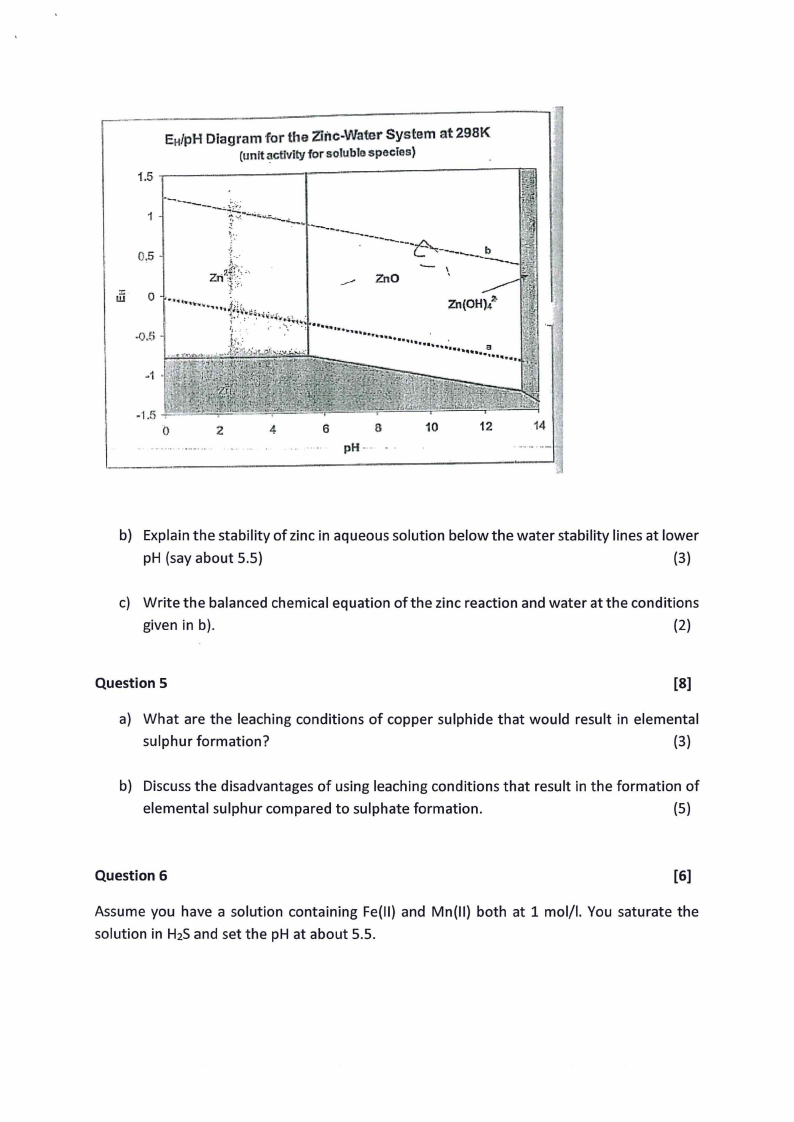
E1.tfpHDiagram for the Zinc-Water System at 298K
(unit actfvit,J for solubto species)
.
1.5 ....--------.---------7mn
0,5
-----~-----------~-- ----b
-\\
Jj
0
2
4
6
8
10
12
14
pH-- - .
b) Explain the stability of zinc in aqueous solution below the water stability lines at lower
pH (say about 5.5)
(3)
c) Write the balanced chemical equation of the zinc reaction and water at the conditions
given in b).
(2)
Question 5
[8]
a) What are the leaching conditions of copper sulphide that would result in elemental
sulphur formation?
(3)
b) Discuss the disadvantages of using leaching conditions that result in the formation of
elemental sulphur compared to sulphate formation.
(5)
Question 6
[6]
Assume you have a solution containing Fe(II) and Mn(II) both at 1 mol/1. You saturate the
solution in H2Sand set the pH at about 5.5.
 |
3 Page 3 |
▲back to top |
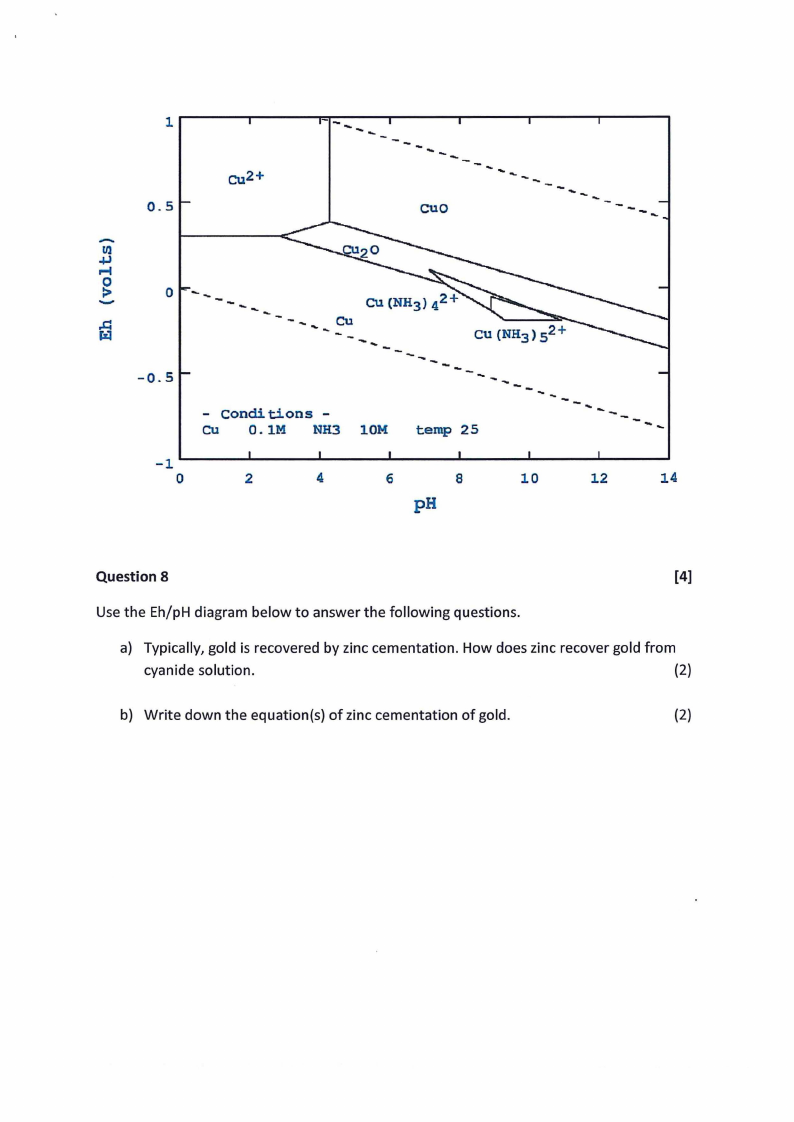
1
cu2+
0.5
-l/l
+l
r-4
-0 >
0-
.c:
iz:I
---
....
.... ...
cuo
-0.5
- Conditions -
...-
cu O.lM NH3 10M temp 25
-1
0
2
4
6
8
10
12
14
pH
Question 8
[4]
Use the Eh/pH diagram below to answer the following questions.
a) Typically, gold is recovered by zinc cementation. How does zinc recover gold from
cyanide solution.
(2)
b) Write down the equation(s) of zinc cementation of gold.
(2)
 |
4 Page 4 |
▲back to top |

b) What is the flow rate of resin in the elution column?
(4)
c) What is the acid concentration in the barren solution from electrowinning?
(8)
d) What is the effect on the electrowinning operation of the small amounts of iron eluted
from the resin? Suggest how the situation should be handled on the plant.
(4)
9.2 Discussthe factors that determine the operating current density in copper electrorefining.
(5)
 |
5 Page 5 |
▲back to top |
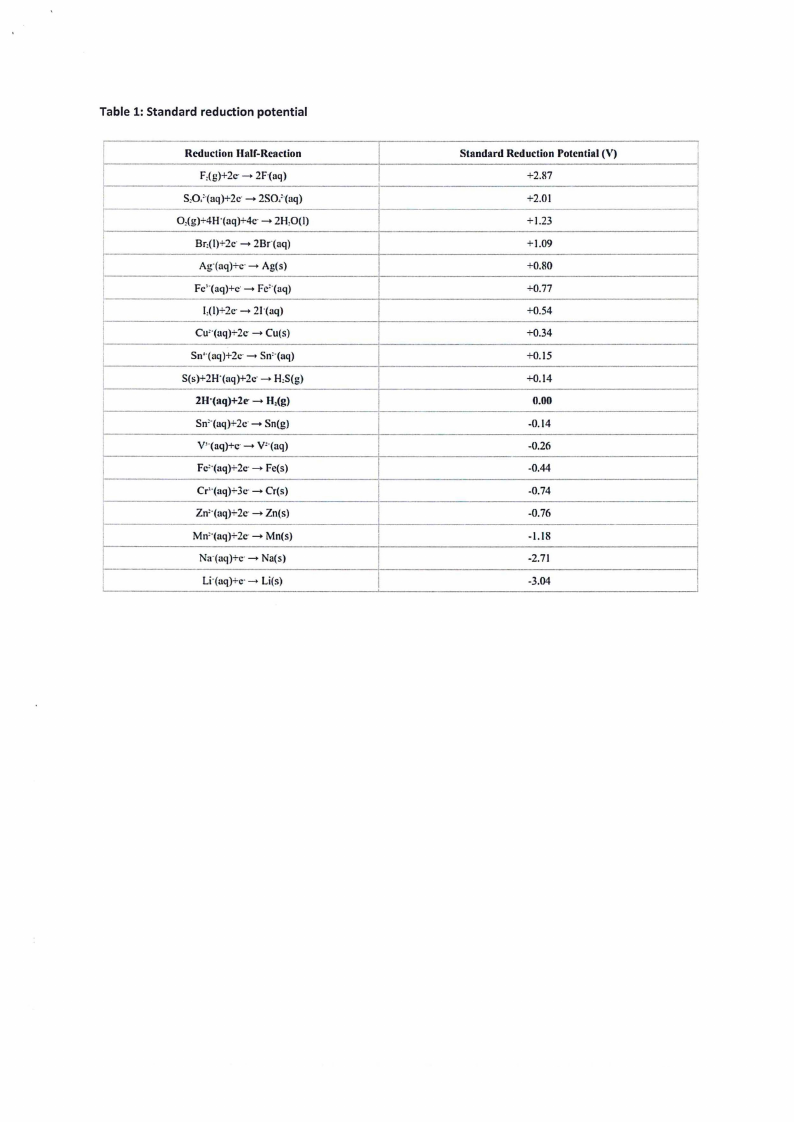
Table 1: Standard reduction potential
Reduction Hair-Reaction
F,(g)+2c--+ 2F·(aq)
S,O/(aq)+2c·-+ 2SO,'-(aq)
O,(g)+4H·(aq)+4c·-+ 2H,O(I)
Br,(1)+2c--+ 2Br·(aq)
Ag·(aq)+c·-+ Ag(s)
Fc'·(aq)+c·-+ Fe'·(aq)
'
l,(1)+2c--+ 21·(aq)
Cu=·(aq)+2c--+ Cu(s)
Sn'·(aq)+2c -+ Sn=·(aq)
S(s)+2H"(aq)+2c--+ H,S{g)
2H·(aq)+2e--+ H,(g)
Sn'·(aq)+2c·-+ Sn(g)
V1·(aq)+c·-+ v=·(aq)
Fc'·(aq)+2c--+ Fe(s)
Cr'·(aq)+ 3c--+ Cr(s)
Zn'·(aq)+2c·-+ Zn(s)
Mn'·(aq)+2c·-+ Mn(s)
Na·(aq)+c·-+ Na(s)
Li·(aq)+c·-+ Li(s)
Standard Reduction Potential (V)
I
+2.87
+2.01
+1.23
+1.09
+0.80
+0.77
+0.54
+0.34
+0.15
+0.14
0.00
-0.14
-0.26
-0.44
-0.74
-0.76
-1.18
-2.71
-3.04
 |
6 Page 6 |
▲back to top |
The Periodic Table of the Elements
1
H
Hydrogen
1.00794
3
Li
Lithium
6.941
11
Na
Sodium
22.989770
19
K
Potassium
39.0983
37
Rb
Rubidium
85.4678
55
Cs
Cesium
132.90545
87
Fr
Francium
(223)
4
Be
Beryllium
9.012182
12
Mg
Magnesium
24.3050
20
Ca
Calcium
40.078
38
Sr
Strontium
87.62
56
Ba
Barium
137.327
88
Ra
Radium
(226)
21
Sc
Scandium
44.955910
39
y
Yttrium
88.90585
57
La
Lanthanum
138.9055
89
Ac
Actinium
(227)
22
Ti
Titanium
47.867
40
Zr
Zirconium
91.224
72
Hf
Hafnium
178.49
104
Rf
Rutherfordium
(261)
23
V
Vanadium
50.9415
41
Nb
Niobium
92.90638
73
Ta
Tanlalum
180.9479
105
Db
Dubnium
(262)
24 25
Cr Mn
Chromium Manganese
51.9961 54.938049
42 43
Mo Tc
Molybdenum Technetium
95.94
(98)
74
w
Tungsten
183.84
75
Re
Rhenium
186.207
106 107
Sg Bh
Seaborgium
(263)
Bohrium
(262)
26
Fe
Iron
55.845
44
Ru
Ruthenium
101.07
76
Os
Osmium
190.23
108
Hs
Hassium
(265)
27
Co
Cobol!
58.933200
45
Rh
Rhodium
102.90550
77
Ir
Iridium
192.217
109
Mt
Meitnerium
(266)
28
Ni
Nickel
58.6934
46
Pd
Palladium
106.42
78
Pt
Platinum
195.078
110
(269)
29
Cu
Copper
63.546
47
Ag
Silver
107.8682
79
Au
Gold
196.96655
111
(272)
30
Zn
Zinc
65.39
48
Cd
Cadmium
I 12.411
80
Hg
Mercury
200.59
112
5
B
Boron
10.81 I
13
Al
Aluminum
26.981538
31
Ga
Gallium
69.723
49
In
Indium
114.818
81
Tl
Thallium
204.3833
113
6
C
Carbon
12.0107
14
Si
Silicon
28.0855
32
Ge
Germanium
72.61
50
Sn
Tin
I 18.710
82
Pb
Lead
207.2
114
7
N
Nitrogen
14.00674
15
p
Phosphorus
30.973761
33
As
Arsenic
74.92160
51
Sb
Antimony
121.760
83
Bi
Bismulh
208.98038
(277)
8
0
Oxygen
15.9994
16
s
Sulfur
32.066
34
Se
Selenium
78.96
52
Te
Tellurium
127.60
84
Po
Polonium
(209)
9
F
Fluorine
18.9984032
17
Cl
Chlorine
35.4527
35
Br
Bromine
79.904
53
I
Iodine
126.90447
85
At
Astatine
(210)
2
He
Helium
4.003
10
Ne
Neon
20.1797
18
Ar
Argon
39.948
36
Kr
Krypton
83.80
54
Xe
Xenon
131.29
86
Rn
Radon
(222)
58
Ce
Cerium
140.116
90
Th
Thorium
232.0381
59 60
Pr Nd
Praseodymium Neodymium
140.90765 144.24
91 92
Pa u
Protactinium Uranium
231.03588 238.0289
61
Pm
Promethium
(145)
93
Np
Neptunium
(237)
62
Sm
Samarium
150.36
94
Pu
Plutonium
(244)
63
Eu
Europium
151.964
95
Am
Americium
(243)
64
Gd
Gadolinium
157.25
96
Cm
Curium
(247)
65
Tb
Terbium
158.92534
97
Bk
Derkdium
(247)
66
Dy
Dysprosium
162.50
98
Cf
Colifornium
(251)
67
Ho
Holmium
164.93032
99
Es
Einsteinium
(252)
68
Er
Erbium
167.26
100
Fm
Fcnnium
(257)
69
Tm
Thulium
168.93421
101
Md
Mendt:lt:vium
(258)
70
Yb
Ytterbium
173.04
102
No
Nobi:lium
(259)
71
Lu
Lutetium
I 74.967
103
Lr
Lowrencium
(262)
1995 JUPAC masses ond Approved Names from ht1p://www.ehrnupnw.nc.uk/iupru;/A1\\Vt/
masses for l07-111 from C&EN, Murch 13. 1995.p. 35
112 from http://www.gsi.de/zl 12c.html





