 |
HSC511S - HEALTH SCIENCE CHEMISTRY - 1ST OPP - JUNE 2023 |
 |
1 Page 1 |
▲back to top |

nAm I BIA Un!VE RS ITY
OF SCIEnCE Ano TECHnOLOGY
FACULTYOF HEALTH,APPLIEDSCIENCESAND NATURALRESOURCES
SCHOOLOF HEALTHSCIENCES
DEPARTMENT OF CLINICALHEALTHSCIENCES
QUALIFICATION: BACHELOROF MEDICALLABORATORYSCIENCES
BACHELOROF HEALTHSYSTEMSINFORMATION MANAGEMENT
BACHELOROF ENVIRONMENTHEALTHSCIENCES
BACHELOROF HUMAN NUTRITION
QUALIFICATIONCODE: 08BMLS
07BHIS
08BEHS
LEVEL: 5
08BOHN
COURSECODE: HSCSllS
COURSENAME: HEALTHSCIENCECHEMISTRY
SESSION:
JUNE 23
PAPER:
THEORY
DURATION: 3 HOURS
MARKS:
100
FIRSTOPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S)
DR MPINGANA AKAWA
MODERATOR:
DR MARIUS MUTORWA
INSTRUCTIONS
1. Answer ALL the questions.
2. Write clearly and neatly.
3. Number the answers clearly.
PERMISSIBLEMATERIALS
1. Pen
2. Non-programmable calculator
THIS QUESTION PAPERCONSISTSOF 9 PAGES(Including this front page and the periodic
table)
 |
2 Page 2 |
▲back to top |

SECTION A
[60]
QUESTION 1: Multiple Choice Questions
• There are 20 multiple choice questions in this section. Each question carries 3 marks.
• Answer ALLquestions by selecting the letter of the correct answer.
• Choose the best possible answer for each question, even if you think there is another
possible answer that is not given.
1.1 The number of significant figures in 0.010:
A. is 4
B. is 3
C. is 2
D. is 1
1.2 Write the following number 0.000004013 using scientific notation.
A. 4.013 x 10-6
B. 4.013
C. 4.013 X 106
D. 4.01 x 107
1.3 Do the following calculation and give the answer to the correct number of significant
figures?
A. 0.36
B. 3.6
2.568 X 5.8
4.186
C. 3.558
D. 0.6
1.4 A toddler with a fever has a temperature of 103° F. What is this temperature reading
in Celsius?
A. 39.4° C
B. 37.1° C
C. 42.7° C
D. 35.3° C
2
 |
3 Page 3 |
▲back to top |

1.5 List the following ions in order of decreasing ionic radius:N 3-, Na+, F-,Mg 2+, 0 2-.
A. Na+, Mg 2+, N3-, 0 2-, F-
B. Mg2+, Na+, F-,0 2-, N3-
C. F-,0 2-, N3-, Mg 2+, Na+
D. Mg2+, Na+, N3-, 0 2-, F-
1.6 Give the full electron configuration
A. 1s2 2s2 2p6 3s23p64s2
B. 1s2 2s2 2p6 3s23p6
C. 1s1 2s2 2p6 3s23p6 4s1
D. 1s2 2s2 2p5 3s2 3p6
of the following
element:
ca+2.
1.7 Balance the following equation by providing the missing coefficients:
_Na2SiO3's) + _HF (aq) H2SiFG(aq) + _NaF(aq) + _H2O(I)
A. 1, 8, 2, 3
B. 2, 6, 2, 3
C. 1, 8, 1, 2
D. 2, 4, 3, 2
1.8 How many moles are in 4.6 x 1024of sulfur atoms?
A. 2.8 moles
B. 7.6 moles
C. 6.7 moles
D. 76.0 moles
1.9 How many grams of Na2SO4,are required to make 0.350 L of 0.500 M Na2SO4?
A. 24.9 g Na2SO4
B. 23.4 g Na2SO4
C. 34.9 g Na2SO4
D. 28.9 g Na2SO4
1.10 Which of the following is the right combination of oxidation numbers for the
following compound: Mn2O1.
A. Mn=+2,O=+7
B. Mn= +14, 0 = -2
C. Mn= +7, 0 = -2
D. Mn= +2, 0 = -7
3
 |
4 Page 4 |
▲back to top |

1.11 From the following list select the elements that are metals:
I. Fe, II. S, Ill. Si, IV. Na, V. U, VI. Hg
A. 11,111
B. I, 111I,V, V,
C. I, IV, V, VI
D. Ill, IV, V
1.12 How many moles are there in 24.0g of C?
A. 4.1 moles C
B. 2.0 moles C
C. 3.2 moles C
D. 3.4 moles C
1.13 How many molecules are in 0.63 moles of molecules?
A. 8.3 x 1021molecules
B. 4.1 x 1026molecules
C. 3.8 x 1024molecules
D. 3.8 x 1023molecules
1.14 How many liters are required to make 800ml of a 2.0M H2SO4solution, starting
with a 6.0M stock solution?
A. 26.1 l
B. 0.62 l
C. 0.26 l
D. 12.4 l
1.15 Which one of the following name-formula combinations is NOT correct?
A. Mercury (I) nitrate, HgNO3
B. Calcium phosphate, Ca3(PO4)2
C. Copper (II) sulfate pentahydrate, CuSQ4·5H2O
D. Hydrofluoric acid, HF(aq)
1.16 If 10 ml of 1 M HCI was required to titrate a 20 ml of NaOH solution of
unknown concentration to its endpoint, what was the concentration of the NaOH?
A. 0.5 M
B. 1 M
C. 1.5 M
D. 2 M
4
 |
5 Page 5 |
▲back to top |

1.17 What is the percentage composition of calcium in calcium hydroxide, Ca(OH)2?
A. 40%
B. 43%
C. 54%
D. 69%
E. 74%
1.18 Which of these would be least soluble in water?
A. Ethanol (CH3CH2-OH)
B. Butanol (CH3CH2CH2CH2-OH)
C. Pentanol (CH3CH2CH2CH2CH2-OH)
D. Hexanol (CH3CH2CH2CH2CH2CH2-OH)
E. Octanol (CH3CH2CH2CH2CH2CH2CH2CH2-OH)
1.19 The alcohol shown below is a:
(CH3)2CHOH
A. Primary alcohol
B. Secondary alcohol
C. Tertiary alcohol
D. Allylic alcohol
1.20 What is the name of the following alkene according to the IUPAC rules?
CH2-CH3
-! CH3- CH2 = CH - CH3
A. 2-ethyl-3-pentene
B. 3-ethyl-2-pentene
C. 3-methyl-2-pentene
D. 3- pentene -2- ethyl
END OF SECTIONA
s
 |
6 Page 6 |
▲back to top |
SECTION B
[40]
QUESTION 2
2.1 Compute the following and report answers to correct number of significant figures and
answer should be in scientific notation.
[3]
a) (0.62 +0.532) - 0.5
b) (3.250 x 102) x (2.30 x 104)
C) 0.000440 X 17.22 + 203,000
QUESTION 3
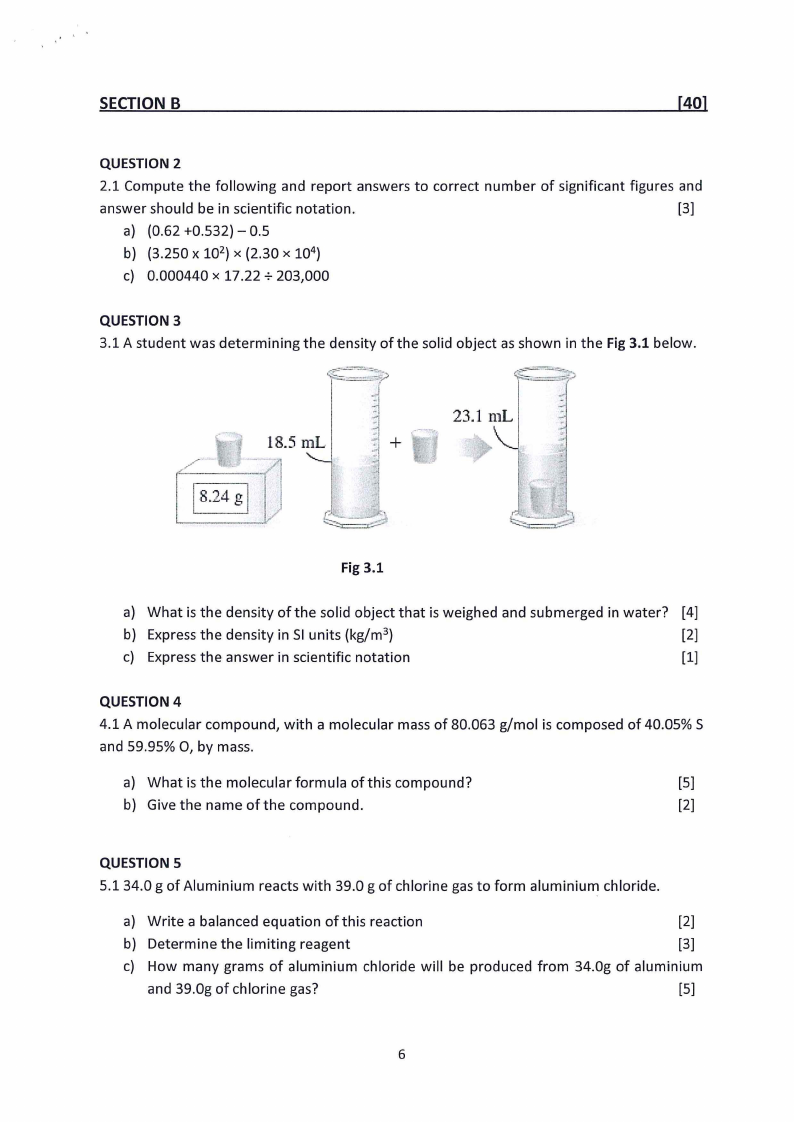
3.1 A student was determining the density of the solid object as shown in the Fig 3.1 below.
18.5 mL
23.l mL
-
+
Fig 3.1
a) What is the density of the solid object that is weighed and submerged in water? [4]
b) Express the density in SI units (kg/m 3)
[2]
c) Express the answer in scientific notation
[1]
QUESTION 4
4.1 A molecular compound, with a molecular mass of 80.063 g/mol is composed of 40.05% S
and 59.95% 0, by mass.
a) What is the molecular formula of this compound?
[5]
b) Give the name ofthe compound.
[2]
QUESTION 5
5.134.0 g of Aluminium reacts with 39.0 g of chlorine gas to form aluminium chloride.
a) Write a balanced equation of this reaction
[2]
b) Determine the limiting reagent
[3]
c) How many grams of aluminium chloride will be produced from 34.0g of aluminium
and 39.0g of chlorine gas?
[5]
6
 |
7 Page 7 |
▲back to top |

QUESTION 6
6.1 For the reaction of aqueous silver nitrate (AgN03) solution and aqueous sodium iodide
(Nal) solution write the balanced:
a) Molecular equation of the reaction
[3]
b) The complete ionic equation of the reaction
[3]
c) The net ionic equation of the reaction
[2]
QUESTION 7
7.1 For each of the following, give the corresponding name or formula
[5]
a) Copper {I) sulphate
b) Dichlorine heptoxide
c) Co3(P04)2
d) P4SG
e) chromium (VI) oxide
END OF THE EXAMINATION QUESTIONS
7
 |
8 Page 8 |
▲back to top |
1
[¾]2
3 u!-.!.
U+I
Periodic Table of the Elements
18
13
14
15
16
17
I'~I ~ C
6 7 ~~LJO ~n
W12.0l I
N
N"~n
1-4.007
F
Fbon,e
18."8
Ne
N<en
20.180
13Al
Si 14
~[1]6
S 17Cl [8 Ar
·iI'i'~l1i3'5hI:':~ ltiJ~i Abnw.im S3icon
3
4
5
6
7
8
9
10
11
12
2ua2
2s.oe4
Cr Mn Fe ~7Co 2sNi ~9Cu ~oZn 31Ga 32Ge
Ch<-omun
Iron
Cob>lt
l'6ckd
Zmc
G>lkn, G~
l::=:=;=i.ie~a=~~;='=":":'==l=¾==J:3~s=i.=i.s.~,s3:.=an=~3=sS=8l:.='"=~=s~a:~=~==/1::~=-=4-8='5~39:==4=9"=.m::==so=n:.l" s1
Sul!llr
n.ou
Oilcme
JS.4Sl
Arpn
n.m
Se ~sBr ~6Kr
~.....
8tcmine
Krypcoo
s278.°'53.,,.904 u484ao
Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
87.42
l;J~s~!
137327
1:I ..rTa~W Re ~Os 67Ir 7Pt 88.'°'
91.224
Nicbun
92.'°'
t1<&denom Tedwit-!ium
'3.'4
'6.,07
101.07
llhoc!un
102.'°'
l">bcwn
106.42
~n4'
7~~4
T:,nt>bm
1eo.,+e
T....pen
193.as
~5
IIMni:m
1eoo1
O>mium
1,o,n
Iridium
1n.22
78
l'hin,m
i,s.oa
Sil.er
107.8'8
79Au
Gold
"'·'"
~8•l1J~3 Ll 112.411
lncSum
114.818
Te
118.71
Al>timoffr Tel\\nrn
121.7'°
12U
locf-
126.904
XffiOII
131.29
g Tl
Bi Po At Rn
crcury
Th>.1ium
&mulh
l'dofti>m
Jutoline
lt,don
,
204.383
20a.,eo £208.'921 209.~
122.01e
JL !!! ti! ~L PJ>m Ra Rf 88
89-103 11·04
10~- ~06
-~
~07
~08
109
110
Ill
Het1!m
112 - 113
~~15~16
~p
22"2S ______J_L £2,11 (2'2]
!™I
[™]
IU'I
(2'8J
(26'1]
(272]
(277)
...,b,o,,,,,
uniuw,-
Li~µ1~17!
£2,eJ .....,_,,
~18
.....,.,_
£: s~58
a.'°' 13
-
~Ho.us
~
232.038
59
~61
62
63
~lri]s
~:so.3, ~<ffl
9;-t0.'°~32144.24 ~~4,u13
&.E~l!m
9~s,."4 96
~,e,m-~~ p!,aNII
~ffl
231.03'
1311.02' 237.048 244.064 'Hl.061
247.070
~67
68
!!! !f~ !!: !!!! !!!. oEJAffl
~;m H 9;sa.ns
981"1.YJ ~1'4.'30
69
10
31
1;~·'
y~
3
1~; r>4
4
03 -"'
!l ~£t~ ~!!<L U~~-
247..070 251.CleO [lS4J
1S7.0,S _ _ 2SIJ.1~ _ 2S,.101 _ (2'2)





