 |
IBC521S - INTRODUCTION TO BIOCHEMISTRY - 1ST OPP - NOVEMBER 2023 |
 |
1 Page 1 |
▲back to top |

nAm I BIA un IVE RS ITV
OF SCI En CE
TECH no LOGY
Faculty of Health, Natural
Resources and Applied
Sciences
School of Health Sciences
Department of Preventative
Health Sciences
QUALIFICATION: BACHELOR OF HUMAN NUTRITION
QUALIFICATION CODE: 0SBOHN
COURSE: INTRODUCTION TO BIOCHEMISTRY
DATE: NOVEMBER 2023
DURATION: 3 HOURS
13 Jackson Kaujeua
Private Bag 13388
Windhoek
NAMIBIA
Street
T: +264 61 207 297
F: +264 61 207 997
E: dphs@nust.na
W: www.nust.na
LEVEL: 5
COURSE CODE: IBC521S
SESSION: 1
MARKS: 100 MARKS
FIRST OPPORTUNITY: QUESTION PAPER
EXAMINER:
MODERATOR:
Mr Junias Natangwe Jackson
Mr George Waliomuzibu Mukisa
INSTRUCTIONS:
11. Answer all questions on the separate answer sheet.
12. Please write neatly and legibly.
13. Do not use the left side margin of the exam paper. This must be allowed for the
examiner.
14. No books, notes and other additional aids are allowed.
15. Mark all answers clearly with their respective question numbers.
PERMISSIBLE MATERIALS:
Non-Programmable Calculator
ATTACHMENTS:
None
This paper consists of 6 pages including this cover
 |
2 Page 2 |
▲back to top |

rsEcT10NAT°MurnPI'Ec:Ho1d AND TRUE/ FA~._..--
. _ .. _ ( 2~ MARK~
QuEsT1ON1: MULTIPLECHOICEQUESTIONS
(10 MARKS]
Evaluate the statements in each numbered section and select the most appropriate answer or
phrase from the given possibilities. Fill in the appropriate letter next to the number of the
correct statement/phrase on your ANSWERSHEET.
[10)
1.1. Enzymes are biological catalysts that enhance the rate of a reaction by:
a) Decrease the activation energy.
b) Decreasing the amount of free energy released.
c) Increasing the activation energy.
d) Increasing the energy of the transition state.
1.2. The bacterium E. coli require simple organic molecules for growth and energy, it is therefore
a:
a) Chemoautotroph.
b) Chemoheterotroph.
c) Photoautotroph.
d) Photoheterotroph.
1.3. Stereoisomers that are non-superimposable mirror images of each other are known as:
a) Anomers.
b) Diastereomers.
c) Enantiomers.
d) Geometric isomers.
1.4. If the free energy change (tiG) for a reaction is -50.45 kJ/mol, the reaction is:
a) Exothermic.
b) Exergonic.
c) Endergonic.
d) At equilibrium.
1.5. A protein has a tertiary structure formed by interactions between the side chains of the
following pairs of amino acids. For each pair, identify the strongest type of interaction between
these amino acids:
a) Aspartic acid and lysine
b) Phenylalanine and alanine
c) Serine and lysine
d) Two cysteines
1.6. Protein kinases are responsible for transferring which group:
a) oxygen
b) carbon
c) amino
d) phosphate
1.7. Sphingolipids.
a) are made from glycerol
b) are considered non-polar lipids
c) are usually not found in lipid bilayers
d) None of the above
Biochemistry/ introduction to biochemistry (B1O521S/IBC521S)
2
1st Opportunity November 2023
 |
3 Page 3 |
▲back to top |

1.8. Acid-catalyzed hydrolysis of one mole of sucrose gives one mole of glucose and one mole of
fructose. Which compound(s) is (are) responsible for the positive Benedict's Test that one
obtains after hydrolysis of sucrose:
a) Glucose
b) Fructose
c) Glucose and fructose
d) Sucrose, glucose, and fructose
1.9. For glycolysis to produce ATPwhen no oxygen is present, it is necessaryfor cells to convert
_____
to ____
_
a) pyruvate to glucose
b) NADH to ATP
c) ATPto NAO+
d) NADH to NAO+
1.10. Which component is found in all sphingolipids:
a) a carbohydrate
b) a negative charge
c) a phosphate groups
d) an amino alcohol
QUESTION 2: TRUE/FALSE QUESTIONS
[10 MARKS]
Evaluate the statements and select whether the statement is true or false. Write the word 'True'
or 'False' next to the corresponding number on your ANSWERSHEET.
(10)
2.1 A zymogen, is an inactive precursor of an enzyme.
2.2 Polarity allows water molecules to form hydrogen bonds with each other.
2.3 When an ionic compound is dissolved in water, each ion is surrounded by a sphere of
water molecules called a hydration shell ionic compound.
2.4 In isoelectric focusing, proteins are separated based on pH.
2.5 Heterotropic effects are allosteric interactions that occur when substances such as inhibitor
and substrate are bound to the protein.
2.6 The liver is the source of ketone bodies.
2.7 Products from the glycolysis include 6 ATP molecules.
2.8 The cleavage of fructose 1,6-bisphosphate yields two molecules of glycaldehyde-3-
phosphate.
2.9 TCA cycle is also known as Krebs cycle.
2.10 Diffusion of a substance across a membrane is considered active transport.
Biochemistry/ introduction to biochemistry (810521S/IBC521S)
3
1'1 0pportunity November 2023
 |
4 Page 4 |
▲back to top |

~CTION B: SH?RT/LONG ANSWER QUESTIONS
(80MARKS}
Please answer All of the questions in this section.
QUESTION 3
[41]
3.1 Distinguish between the lock-and-key model and induced-fit model of enzyme action. (4)
3.2 What type of interaction would occur between each group present on a substrate molecule
and a functional group of the active site in an enzyme?
(4)
a) -COOH
b) -NH3+
c) -oH
d) -CH(CH3)2
3.3 What is the difference between a cofactor and a coenzyme?
(2)
3.4 In what ways does a competitive inhibitor differ from a noncompetitive inhibitor?
(4)
3.5 Compare the structural elements of glycerophospholipids and sphingolipids. What building
blocks are they made from?
(S)
3.6 Differentiate between gram-negative and gram-positive bacteria, with regards to cell well
structure
(4)
3.7 Define the following terms:
(8)
a) Gluconeogenesis
b) Metabolic alkalosis
c) Vmax in enzyme kinetic
d) Catabolism
3.8 Name the 4 lipid components found in biological membranes
(4)
3.9 Discuss briefly the various enzymes, intermediates products involved in fatty acid
biosynthesis
(6)
QUESTION 4: CALCULATIONS
(15]
4.1 If the [W] is 2.1 x 10-12 M HCIO4,what is the pH? Is the solution acidic, basic or neutral?
(Show all your workings).
(3)
4.2 A buffer solution has a pH of 4.87. if the buffer contains a weak acid with pKa of 4.48,
calculate the ration of the concentration of conjugate base and acid required, (show all you
calculation).
(5)
4.3 Patient Xis a 22-year-old female admitted with complaints of recurrent vomiting for days.
Her laboratory finding includes the following: Na+133, c1·94, Glucose 720 mmol/l, and
HCO3·11.
a) Calculate the anion gap (in mEq/l) of patient X
(4)
b) Does the anion gap suggest that the patient has metabolic acidosis or metabolic
alkalosis. Given a reason for your answer.
(3)
QUESTION S: ENZYMES
(12]
Below is a reaction coordinate diagram. Answer the questions that follows.
Biochemistry/ introduction to biochemistry (B1O5215/IBC5215)
4
1st opportunity November 2023
 |
5 Page 5 |
▲back to top |
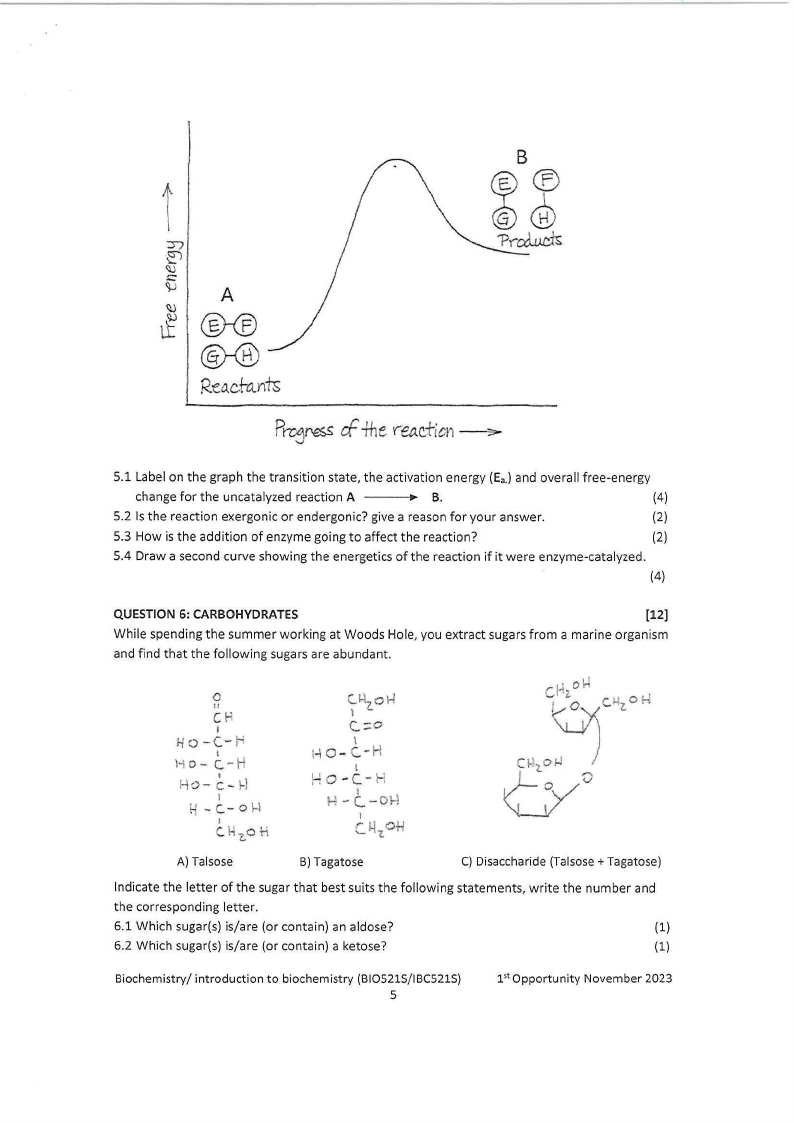
t
A
®-®
®-®
Rea.c.t-a.nts
5.1 Label on the graph the transition state, the activation energy (Ea.)and overall free-energy
change for the uncatalyzed reaction A ---
B.
(4)
5.2 Is the reaction exergonic or endergonic? give a reason for your answer.
(2)
5.3 How is the addition of enzyme going to affect the reaction?
(2)
5.4 Draw a second curve showing the energetics of the reaction if it were enzyme-catalyzed.
(4)
QUESTION 6: CARBOHYDRATES
[12]
While spending the summer working at Woods Hole, you extract sugars from a marine organism
and find that the following sugars are abundant.
0
II
CH
I
Ho-C-1-4
l
Ho- c-H
1
Ho- c-1-l
t
½ - c- o H
l
C.H-z_oH
C..1-olzH..
cl .:.:o
\\
1-10-C.-H
I
Ho-C-H
l
H-C..-OH
I
C 1-lzo+-i
A) Talsose
8) Tagatose
C) Disaccharide(Talsose+ Tagatose)
Indicate the letter of the sugar that best suits the following statements, write the number and
the corresponding letter.
6.1 Which sugar(s) is/are (or contain) an aldose?
(1)
6.2 Which sugar(s) is/are (or contain) a ketose?
(1)
Biochemistry/ introduction to biochemistry (BIO521S/IBC521S)
5
1st Opportunity November 2023
 |
6 Page 6 |
▲back to top |

6.3 Which sugar(s) is/are (or contain) a pentose?
(1)
6.4 Which sugar(s) is/are (or contain) a D sugar?
(2)
6.5 Which sugar(s) is/are a reducing sugar?
(2)
6.6 Which sugar(s) is/ are epimers, if any.
(1)
6.7 Which sugar(s) is/are drawn in the form most likely to be found in the organism?
(1)
6.8 How many stereoisomers would Talsose and Tagatose give rise to?
(3)
---------------------------------------------------.-..-..-.-..-..-..·..-..-..-..-..-..-..-..-..-............................................................................................
END OF QUESTION PAPER
Biochemistry/ introduction to biochemistry (BI0521S/IBC521S)
6
1'' Opportunity November 2023





