 |
GNC501S - GENERAL CHEMISTRY 1A - 1st Opp - JUNE 2022 |
 |
1 Page 1 |
▲back to top |

p
NAMIBIA UNIVERSITY
OF SCIENCE AND TECHNOLOGY
FACULTY OF HEALTH, APPLIED SCIENCES AND NATURAL RESOURCES
DEPARTMENT OF NATURAL AND APPLIED SCIENCES
QUALIFICATION: BACHELOR OF SCIENCE
QUALIFICATION CODE: 07BOSC
LEVEL: 5
COURSE CODE: GNC501S
COURSE NAME: GENERAL CHEMISTRY 1A
SESSION: JUNE 2022
PAPER: THEORY
DURATION: 3 HOURS
MARKS: 100
FIRST OPPORTUNITY EXAMINATION
EXAMINER(S)
DR. EUODIA HESS
MOpERATOR: | OR. MARIUS MUTORWA
QUESTION
PAPER
INSTRUCTIONS
Answer ALL the questions.
Write clearly and neatly.
Number the answers clearly
All written work must be done in blue or black ink and sketches can
be done in pencil
5. No books, notes and other additional aids are allowed
PERMISSABLE MATERIALS
Non-programmable calculators
ATTACHMENTS
1. List of useful constants
2. Periodic Table
THIS QUESTION PAPER CONSISTS OF 8 PAGES
(Including this front page, list of constants and periodic table)
 |
2 Page 2 |
▲back to top |

SECTION A
[60]
QUESTION 1: Multiple Choice Questions
[60]
There are 20 multiple choice questions in this section. Each question carries
3 marks.
Answer ALL questions by selecting the letter of the correct answer.
Choose the best possible answer for each question, even if you think there is
another possible answer that is not given.
When naming a transition metal ion that can have more than one common ionic charge, the
numerical value of the charge is indicated by a:
A. Prefix
B. Suffix
C. Roman numeral following the name
D. Superscript after the name
In which of the following are the symbol and name for the ion given correctly?
A. Fe?* ferrous ion
B. Sn?* tin (III) ion
C. Co** cobaltous ion
D. Pb** lead ion
What is the correct name for Sn3(POq)2?
A. tritin diphosphate
B. tin(Ill) phosphate
C. tin(Il) phosphate
D. tin(IV) phosphate
What is the correct formula for calcium dihydrogen phosphate?
A. Ca(H2POa)2
B. CazH2PO4
C. CazH2HPO,
D. Caz(H2PO.)
Which one of the following Name-Formula combinations is NOT correct (is FALSE)?
A. Mercury (I) chloride, Hg2Cla
B. Dinitrogen trioxide, N2O3
C. Hydrogen chloride, HCl
D. Cerium (IV) phosphate, Ce4(PO.)3
Page 2 of 8
 |
3 Page 3 |
▲back to top |

For a particular organic compound, which of the following pairs can represent the empirical
and the molecular formulas, respectively?
A. CH and CH,
B. CH and CeHe
C. CH2 and C2H2
D. CH2 and C2H3
The percent manganese in potassium manganate, K2MnQOz, is:
A. 13.2%
B. 27.9%
C. 29.0%
D. 34.8%
What external pressure must be supplied to compress 2.76 L of a gas at 298K and 0.878 atm
to 2.00 L at 298K?
A. 484 mmHg
B. 921 mmHg
C. 760 mmHg
D. 878 mmHg
At STP, 4 moles of CO2 gas occupies:
A. 20.4L
B. 22.4L
C. 89.6L
D. 2.24L
10. If 0.250 mol of He(g), 0.500 mol of Ne(g) and 0.150 mol of Ar(g) are transferred to a
previously empty 5.00 L container at 25°C, what is the final pressure in the container?
A. 4.40 atm
B. 2.86 atm
C. 5.72 atm
D. 3.81 atm
11. If a mixture of noble gases consists of 0.150 mole of He, 0.450 mole of Ne, and 0.300 mole
of Ar, what is the mole fraction of Ar in this mixture?
A. 0.300
B. 0.500
C. 0.667
D. 0.333
Page 3 of 8
 |
4 Page 4 |
▲back to top |

12. A solution is prepared by dissolving 0.100 mole of HCl in 75.0 g of water. Calculate the mass
percent HCl in this solution.
A. 0.133%
B. 4.64%
C. 4.87%
D. 4.01%
13. To what volume, mL, must 50.0 mL of 3.50 M H2SOx be diluted in order to make 2 M H2SO4?
A. 25
B. 60.1
C. 87.5
D. 93.2
14. A solution is prepared by dissolving 20.0 g of NaOH in 750 g. of water. The molality of this
solution is?
A. 1m
B. 26.7m
C. 0.0267m
D. 0.667 m
15. Calculate the freezing point in °C of a solution containing 0.0100 mole of a non-electrolyte in
100.0 g of water (K+ of water = 1.86 °C/m).
A. -0.186
B. +0.186
C. 0.010
D. -0.010
16. What is the best name for the following compound?
CH3
A. 2-methylcyclohexene
B. 2-methylcyclohexene
C. 1-methylcyclohex-2-ene
D. 3-methylcyclohexene
17. The condensed structural formula for 2,2-dimethylbutane is:
A. CH3C(CH3)2CH2CH3
B. CeHi4
C. CH3CH(CH3)CH(CH3)CH3
D. C3H7
Page 40f 8
 |
5 Page 5 |
▲back to top |

18. Which one of the following is the correct structural formula for cyclohexane?
A. CeéHi2
B. CsHio
C. CeHi4
D. CéHi0
19. Which of the following is the general formula of the alkynes?
A. CnHan
B. CnaHn
C. CyHans2
D
CnHan-2
20. What is the best name for the following compound?
A. 3-methylenehexane
B. 2-propyl-1-butene
C. 4-ethyl-4-pentene
D. 2-ethyl-1-pentene
|
ho
SECTION B:
[40]
There are FOUR questions in this section. Answer all questions. Show clearly, where necessary,
how you arrive at the answer as all working will carry marks.
Question 1
[10]
a) A fertilizer has mass percent composition 20.00 % C, 6.71 % H, 46.65 % N, and 26.64 % O.
What is its empirical formula?
(3)
b) Consider this reaction:
BF; + HO —H;BO;+ HBF,
The reacting mixture contains 0.496 mol BF; and 0.313 mol H,0.
i) Which compound is the limiting reactant?
(4)
ii) How many moles of HBF, can be produced?
(3)
Question 2
[7]
A solution contains 750 g of ethanol (CH;CH.OH) and 85.0 g of sucrose (molar mass = 180 g/mol).
The volume of the solution is 810.0 mL. Determine:
(i) the density of the solution.
(1)
(ii) the mass percent of sucrose in the solution
(2)
(iii) the mole fraction of sucrose.
(2)
(iv) the molality of the solution.
(1)
Page 5 of 8
 |
6 Page 6 |
▲back to top |
(v) the molarity of the solution.
(1)
Question 3
[14]
a) Ethylene glycol CH2(OH)CH2(OH) is a common automobile antifreeze. It is water soluble and
non-volatile (b.p 197°C). Calculate the freezing point of a solution containing 651 g of this
substance 2505 g of water. (Kr = 1.86°C/m)
(7)
b) The average osmotic pressure of seawater is about 30.0 atm at 25 °C. Calculate the molar
concentration of an aqueous solution of sucrose (C12H22011) that is isotonic with seawater.
(4)
c) What are the factors that affect solubility?
(3)
Question 4
[9]
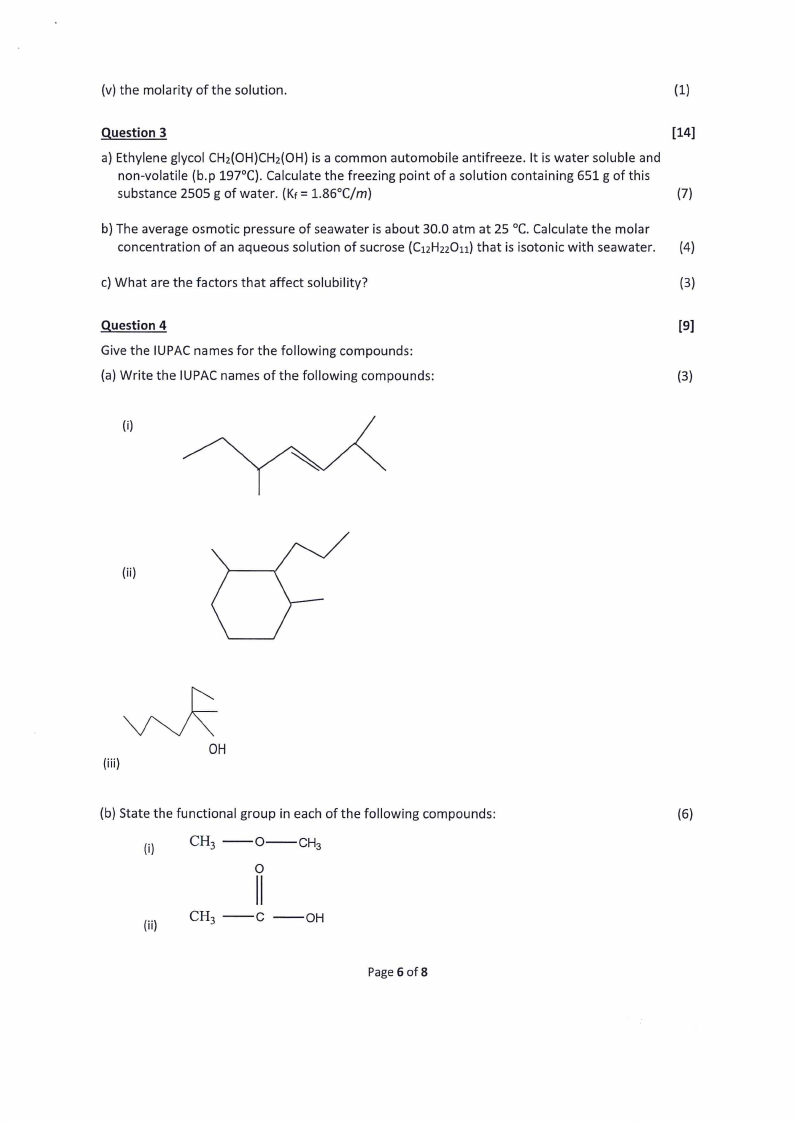
Give the IUPAC names for the following compounds:
(a) Write the IUPAC names of the following compounds:
(3)
(i)
Ns
(ii)
(iii)
(b) State the functional group in each of the following compounds:
(6)
|
(ii)
CH; ——C ——OH
Page 6 of 8
 |
7 Page 7 |
▲back to top |
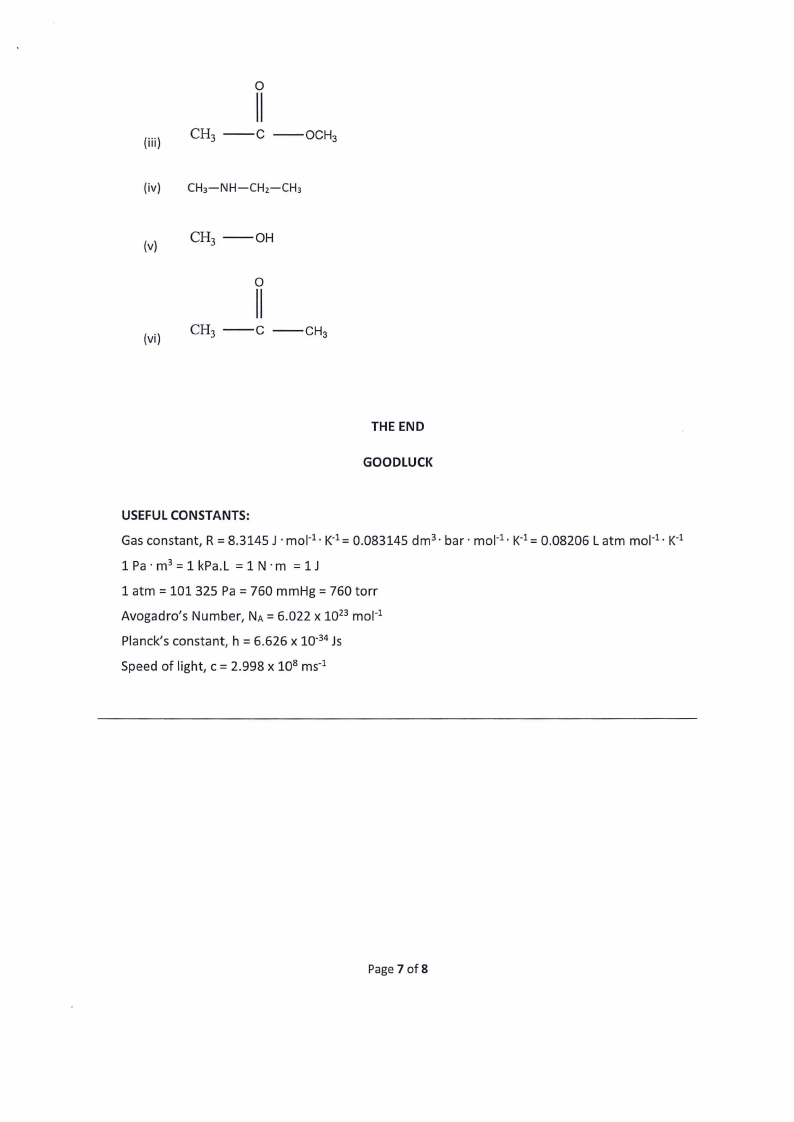
|O
(ii) Cs ——¢ —ovh,
(iv) CH3—NH—CH2—CHs
W)
CH, ——OH
(vi)
CH,
|
—cC ——CH3
THE END
GOODLUCK
USEFUL CONSTANTS:
Gas constant, R = 8.3145 J: mol: K?= 0.083145 dm?: bar: mol: K?= 0.08206 L atm mol?: K?
1Pa:m?=1kPa.L =1N'm =13J
1 atm = 101 325 Pa = 760 mmHg = 760 torr
Avogadro’s Number, Na = 6.022 x 1023 mol?
Planck’s constant, h = 6.626 x 10°34 Js
Speed of light, c = 2.998 x 108 ms+
Page 7 of 8
 |
8 Page 8 |
▲back to top |
Page 8 of 8





