 |
ACS701S - APPLIED COLLOID AND SURFACE CHEMISTRY - 1ST OPP - JUNE 2023 |
 |
1 Page 1 |
▲back to top |
nAm I BI A un IVE RS ITY
OF SCIEnCE AnD TECHnOLOGY
FACULTYOF HEALTH,NATURALRESOURCESAND APPLIEDSCIENCES
SCHOOLOF NATURALAND APPLIEDSCIENCES
DEPARTMENTOF BIOLOGY,CHEMISTRYAND PHYSICS
QUALIFICATION:BACHELOROF SCIENCE
QUALIFICATION CODE: 07BOSC
COURSECODE:ACS701S
SESSION:JUNE 2023
LEVEL:7
COURSENAME: APPLIEDCOLLOID AND SURFACE
CHEMISTRY
PAPER:THEORY
DURATION: 3 HOURS
MARKS: 100
FIRSTOPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S} Prof Habauka M. Kwaambwa
MODERATOR: Prof Edet F. Archibong
INSTRUCTIONS
1. Answer ALL the FIVE questions
2. Write clearly and neatly
3. Number the answers clearly
4. All written work must be done in bule or black ink
5. No books, notes and other additional aids are allowed
6. Mark all answers clearly with their respective question numbers
PERMISSIBLEMATERIALS
Non-programmable Calculators
ATTACHMENT
List of Useful Constants
THIS QUESTION PAPERCONSISTSOF 7 PAGES(Including this front page and List of Useful
Constants)
1
 |
2 Page 2 |
▲back to top |
QUESTION 1
[26]
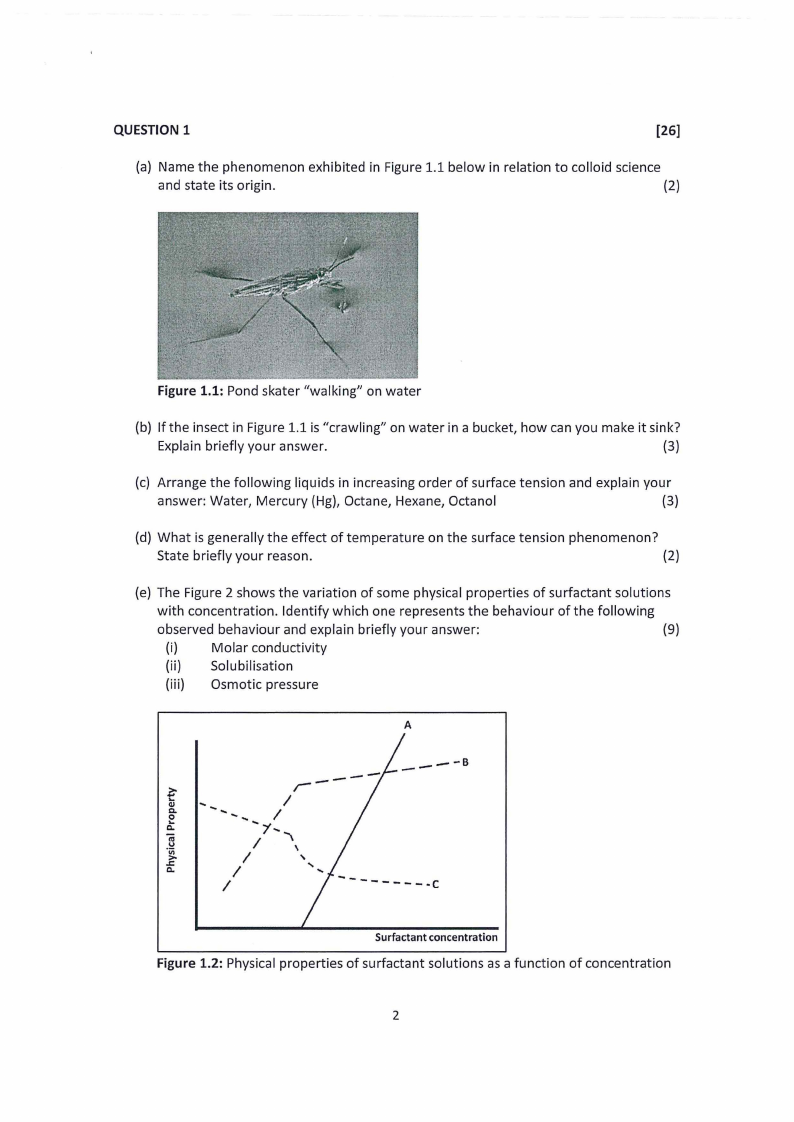
(a) Name the phenomenon exhibited in Figure 1.1 below in relation to colloid science
and state its origin.
(2)
Figure 1.1: Pond skater "walking" on water
(b) If the insect in Figure 1.1 is "crawling" on water in a bucket, how can you make it sink?
Explain briefly your answer.
(3)
(c) Arrange the following liquids in increasing order of surface tension and explain your
answer: Water, Mercury (Hg), Octane, Hexane, Octanol
(3)
(d) What is generally the effect of temperature on the surface tension phenomenon?
State briefly your reason.
(2)
(e) The Figure 2 shows the variation of some physical properties of surfactant solutions
with concentration. Identify which one represents the behaviour of the following
observed behaviour and explain briefly your answer:
(9)
(i)
Molar conductivity
(ii) Solubilisation
(iii) Osmotic pressure
A
,--
.......,.....
/
I
--y
/
...'.'\\
II
' '-..
I
------c
Surfactant concentration
Figure 1.2: Physical properties of surfactant solutions as a function of concentration
2
 |
3 Page 3 |
▲back to top |
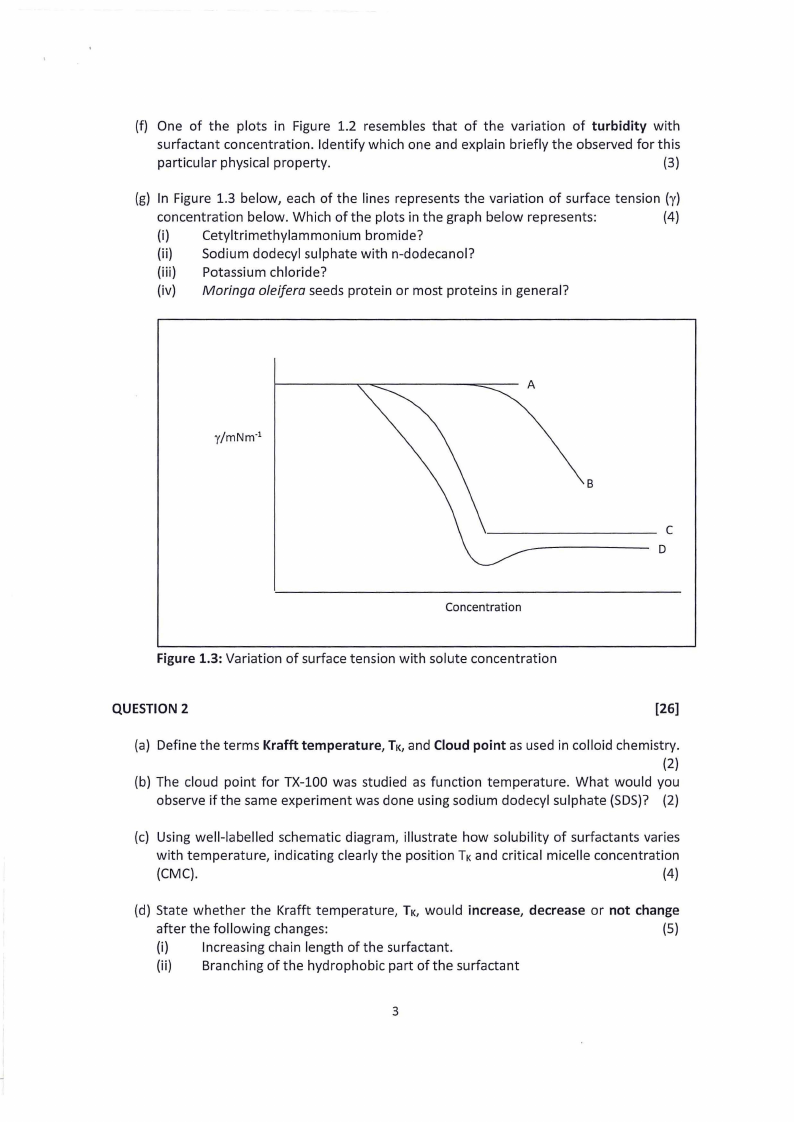
(f) One of the plots in Figure 1.2 resembles that of the variation of turbidity with
surfactant concentration. Identify which one and explain briefly the observed for this
particular physical property.
(3)
(g) In Figure 1.3 below, each of the lines represents the variation of surface tension (y)
concentration below. Which of the plots in the graph below represents:
(4)
(i)
Cetyltrimethylammonium bromide?
(ii) Sodium dodecyl sulphate with n-dodecanol?
(iii) Potassium chloride?
(iv) Moringa o/eifera seeds protein or most proteins in general?
y/mNm· 1
B
C
D
Concentration
Figure 1.3: Variation of surface tension with solute concentration
QUESTION 2
(26]
(a) Define the terms Krafft temperature, TK, and Cloud point as used in colloid chemistry.
(2)
(b) The cloud point for TX-100 was studied as function temperature. What would you
observe if the same experiment was done using sodium dodecyl sulphate (SOS)? (2)
(c) Using well-labelled schematic diagram, illustrate how solubility of surfactants varies
with temperature, indicating clearly the position TKand critical micelle concentration
(CMC).
(4)
(d) State whether the Krafft temperature, TK, would increase, decrease or not change
after the following changes:
(5)
(i)
Increasing chain length of the surfactant.
(ii) Branching of the hydrophobic part of the surfactant
3
 |
4 Page 4 |
▲back to top |

(iii) Addition of NaCl to an ionic surfactant
(iv) Unsaturation (double bonds) of the hydrophobic part of the surfactant
(v) Insertion of EOgroups between alkyl chain and the head group
(e) For a non-ionic surfactant, X, at moderate concentrations, the surface tension (mN/m)
at 298 K,based on the Gibbs adsorption isotherm equation, is given by:
y = 0.62 - 8.4Inc
where c is the concentration in mM.
(i)
Determine the surface excess and area per molecule (A2).
(4)
(ii) Suppose similar experiments were carried out for two other surfactants A and
B, the sign of surface excess obtained for A was opposite to that obtained for
X whereas for Bit was found to be zero. State the type of adsorption exhibited
or implied by each surfactant.
(3)
(iii) If the area of headgroup is 20 A2, comment on the result obtained in (i) above.
(2)
(f) Given a solid (S) and liquid (L), calculate the contact angle, 8, and deduce which of
the following occurs: Perfect wetting, Partial wetting, Non-wetting or Perfectly not
wetted
(4)
(i)
Ys= 18.5 mNm-1, YL= 72.8 mNm-1 and ysL= 91.3 mNm-1
(ii) ys = 307 mNm-1, YL= 45 mNm-1 and YsL= 262 mNm-1
QUESTION 3
[20]
The non-linear form of the Langmuir equation for the adsorption a gas on a solid takes the
form:
= V V111aP
l+aP
(a) State any three assumptions involved in the derivation of the Langmuir adsorption
isotherm equation for molecules at the gas/liquid interface.
(3)
(b) How is this equation modified to account for:
(i)
Competitive adsorption on an adsorbent of two gases A and B without
dissociation?
(2)
(ii) Adsorption of gas A which dissociates into two species?
(2)
(c) Show how the linear form of the Langmuir equation above may be used to determine
the constants.
(5)
(d) One student of mine recently used Moringa seed shells activated carbon to remove
methylene blue (MB) from water, fitted the data to the linear form of the Langmuir
equation for adsorption from solution given below and found the slope and intercept
to be 0.0295 g mg-1 and 0.2573 g2 L-1mg-1, respectively. Determine the values for
monolayer capacity (adsorption capacity) and a in the Langmuir equation under the
experimental conditions used.
(4)
4
 |
5 Page 5 |
▲back to top |
-=C --+-- l
C
q. qma.xa qmax
(e) Under the conditions used in (d) above, determine the specific surface area (in m2g·1)
of the Moringa seed shells activated carbon (Molecular weight of MB is 319.85 g/mol
and the area adsorbed by per molecule of methylene blue is taken A as 130 2•
(4)
QUESTION 4
[28]
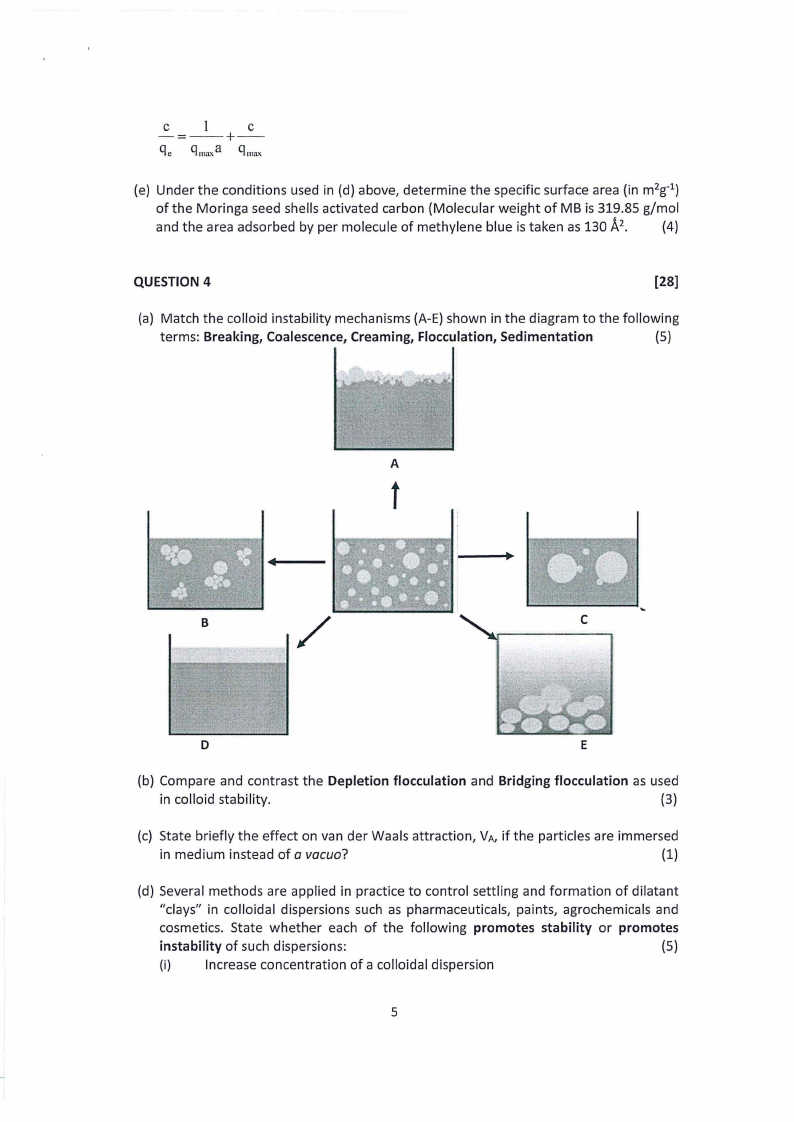
(a) Match the colloid instability mechanisms (A-E) shown in the diagram to the following
terms: Breaking, Coalescence, Creaming, Flocculation, Sedimentation
(5)
A
B
/
C
D
E
(b) Compare and contrast the Depletion flocculation and Bridging flocculation as used
in colloid stability.
(3)
(c) State briefly the effect on van der Waals attraction, VA,if the particles are immersed
in medium instead of a vacuo?
(1)
(d) Several methods are applied in practice to control settling and formation of dilatant
"clays" in colloidal dispersions such as pharmaceuticals, paints, agrochemicals and
cosmetics. State whether each of the following promotes stability or promotes
instability of such dispersions:
(5)
(i)
Increase concentration of a colloidal dispersion
5
 |
6 Page 6 |
▲back to top |

(ii) Viscosity of dispersion medium changed from 10 mPas to 100 mPas
(iii) Prepare colloid particles of size 100 nm instead of 500 nm
(iv) van der Waals forces changed from - 20 kJ/mol to - 100 kJ/mol
(v) Effective composite Hamaker constant of an interacting system of colloidal
particles changed from 4.34 x 10-21 J to 1.28 x 10·20J
(vi) Hamaker constant of the dispersion medium is manipulated such that it is
equal to that of the particles, i.e. Adispersimonedium=Aparticles
(e) The technique of manipulating the Hamaker constant of the dispersion medium to
that of the particles is called contrast matching in Small-angle Neutron Scattering
(SANS). How is this done in practice?
(2)
(f) The WI NGOCwastewater treatment plant in Windhoek is based on a multiple barrier
system and one of stages involves dosing of ferric chloride (FeCl3).What is this stage
called? How is the pH of the water adjusted after this stage?
(2)
(g) There are several mechanisms which colloidal particles acquire charge. Answer the
following questions based on one of the mechanisms.
(i) The solubility product for AgBr is Ksp= [Ag+][sr·]. The AgBr particles in aqueous
dispersion are negatively charged. What does this mean?
(2)
(ii) As a Colloid Scientist, explain how you can manipulate AgBr particles so that you
have a dispersion with zero charged AgBr particles and another with positively
charged AgBr particles.
(2)
(iii) What would the effect of adding KNO3,if any, to negatively charged Agl particles?
Give a reason for your answer.
(2)
(h) Apart from the above mechanism in (g), isomorphous substitution is another
mechanism particles acquire charge. Deduce the resulting charge of clay particles if
metal X (valency= 4+) replaces metal M (valency= 3+)?
(1)
(i) Using combining relations based on the Hamaker constants of pure materials (Ai),
calculate the composite Hamaker constant for interacting system of Mica-Benzene-
Teflon. Comment on the result.
(3)
Given:
Material
Mica
Teflon
Benzene
Ai X 1020 J
13.5
3.8
5.0
END OF EXAM QUESTIONS
6
 |
7 Page 7 |
▲back to top |

USEFULCONSTANTS:
Universal Gas constant
Boltzmann's constant,
Planck's constant
Debye-Huckel's constant,
Faraday's constant
Mass of electron
Velocity of light
Avogadro's constant
1 electron volt {eV)
R=
k=
h=
A=
F=
me =
C
=
NA =
=
8.314 J K·1 mo1·1
1.381 X 10-23 J K·1
6.626 X 10-34 J S
0.509 {mol dm·3) 112 or mol·05 kg05
96485 C mol·1
9.109 X 10-31 kg
2.998 x 108 m s·1
6.022 X 1023
1.602 X 10-19 J
7





