 |
PNM710S - Pyrometallurgy on Non-Ferrous Metals- 1st OPP - JUN 2023 |
 |
1 Page 1 |
▲back to top |

n Am I BI A u n IVE Rs ITY
OF SCIEnCE Ano TECHnOLOGY
FACULTY OF ENGINEERING AND SPATIAL SCIENCES
DEPARTMENT OF MECHANICAL, MINING AND PROCESSENGINEERING
QUALIFICATION: BACHELOR OF ENGINEERING IN METALLURGY
QUALIFICATION CODE: 08BMET
LEVEL: 8
COURSE CODE: PNM710S
COURSE NAME: PYROMETALLURGY OF NON-
FERROUS METALS
SESSION: JUNE 2023
DURATION: 3 HOURS
PAPER: THEORY
MARKS: 100
EXAMINER(S)
FIRST OPPORTUNITY EXAM PAPER
Prof. Godfrey Dzinomwa
MODERATOR:
Prof. Sofya Mitropolskaya
INSTRUCTIONS
1. Answer all questions.
2. Read all the questions carefully before answering.
3. Marks for each questions are indicated at the end of each question.
4. Please ensure that your writing is legible, neat and presentable.
PERMISSIBLE MATERIALS
1. Examination paper.
THIS QUESTION PAPER CONSISTS OF 5 PAGES (Including this front page)
 |
2 Page 2 |
▲back to top |
Question 1
(a) Discuss the advantages of using a Top Submerged Lance (TSL) furnace compared to an
electric furnace in the smelting of copper sulphide concentrates (5 marks).
(b) By applying Stoke's law, derive an expression for the settling velocity Vs of a matte droplet
of density Ps in a molten slag of density Pt in terms of the diameter d and density of the
matte droplet, and the viscosityµ and density of the slag from first principles. Assume that
the rate of settling obeys Stoke's law, and that the frictional force between matte droplets
and slag= 6Vµm (5 marks).
(c) Given that matte density is 5500kg/m 3, slag density is 3500kg/m 3 and slag viscosity is 0.1
kg/m.s, calculate the settling velocities of and the times taken by matte droplets of radii in
mm; 12; 10; 8; 4; 2 settling through 2m of slag? (10 marks).
p(matte) = 5500 kg/m 3
p(slag) = 3500 kg/m 3
µ(slag)= 0.1 kg/m 3
Settling in 2m of slag.
(d) How do matte droplets get entrained in slag during converting and what practical measures
are applied in industry in order to increase the rate at which matte droplets settle out of the
slag (5 marks).
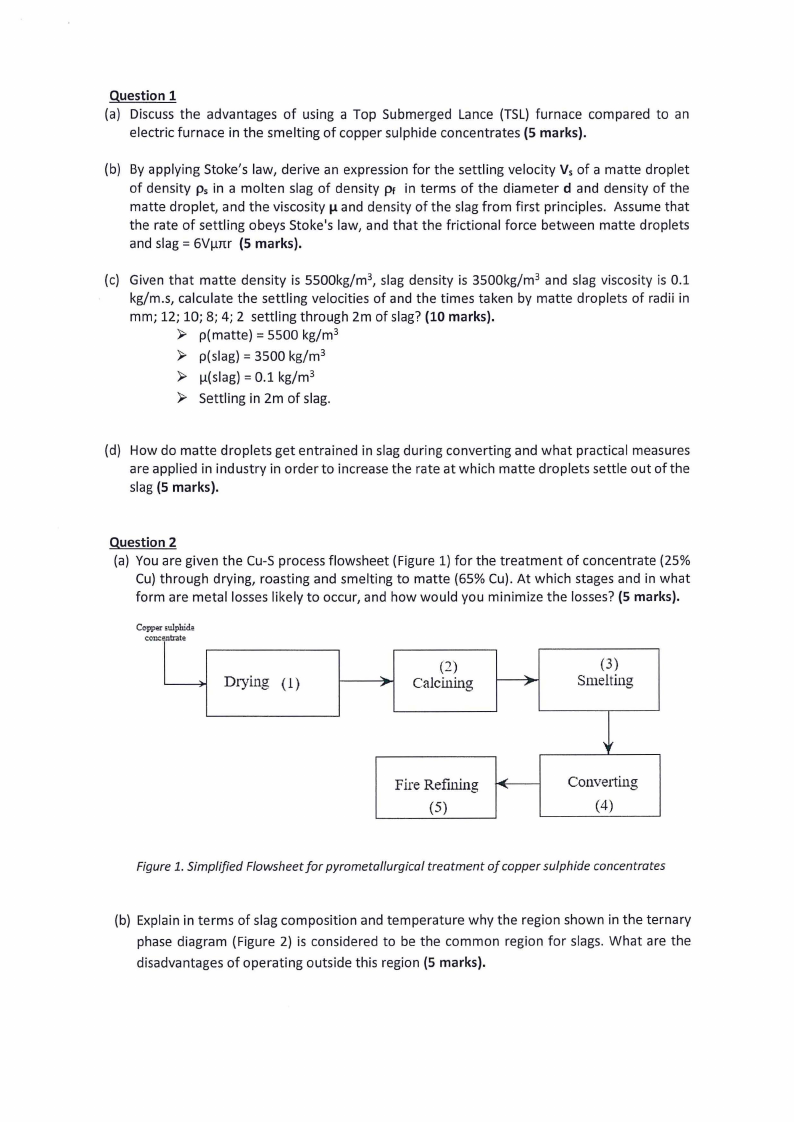
Question 2
(a) You are given the Cu-S process flowsheet (Figure 1) for the treatment of concentrate {25%
Cu) through drying, roasting and smelting to matte {65% Cu). At which stages and in what
form are metal losses likely to occur, and how would you minimize the losses? (5 marks).
Copper ,ulplrida
con:: ntrate
Drying (1)
(2)
(3)
.,. Calcining
r
Smelting
Fire Refining .i...
(5)
"
Converting
(4)
Figure 1. Simplified Flowsheet for pyrometallurgical treatment of copper sulphide concentrates
(b) Explain in terms of slag composition and temperature why the region shown in the ternary
phase diagram (Figure 2) is considered to be the common region for slags. What are the
disadvantages of operating outside this region (5 marks).
 |
3 Page 3 |
▲back to top |
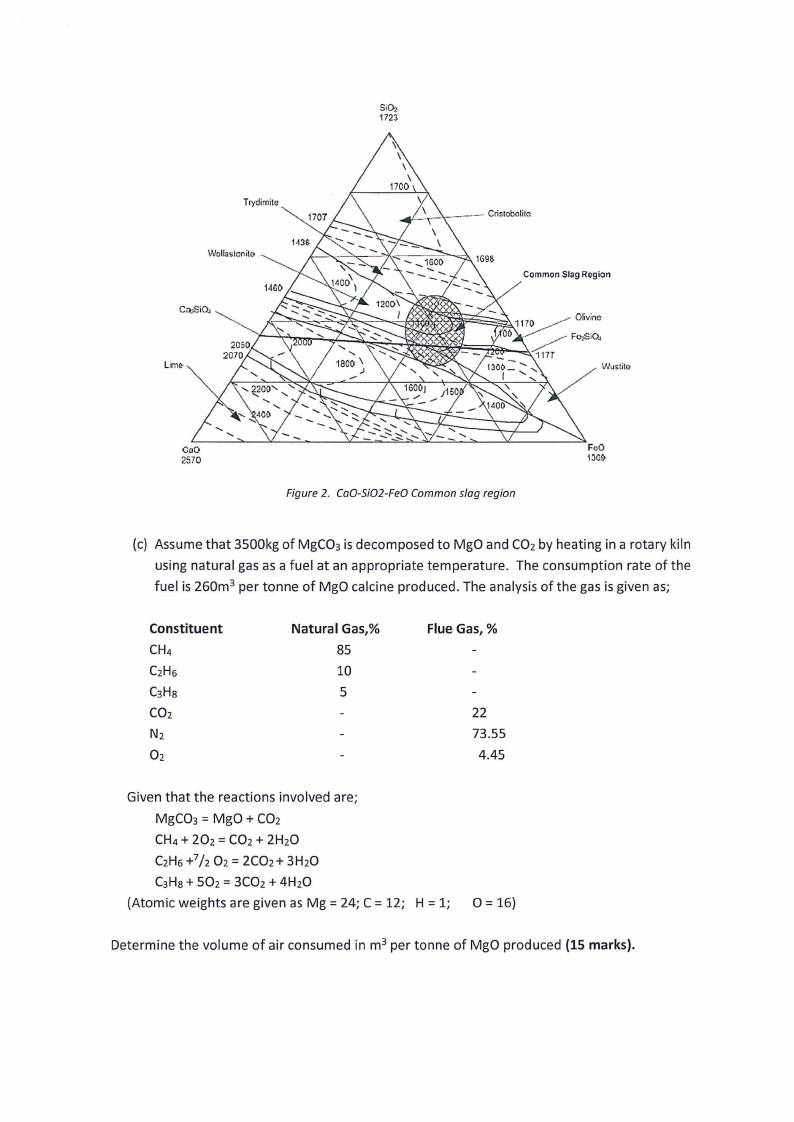
SiO,
172~
Trydimite
Ca.,SiOJ
2050'
2070
Lim~
Common Slag Region
11 70
_.,,,...O.-livine
_,,,--
00 ...--- _,..- F<...,SOi J
coo
FcO
2570
13C~
Figure 2. Ca0-Si02-Fe0 Common slag region
(c) Assume that 3500kg of MgCO3is decomposed to MgO and CO2by heating in a rotary kiln
using natural gas as a fuel at an appropriate temperature. The consumption rate of the
fuel is 260m 3 per tonne of MgO calcine produced. The analysis of the gas is given as;
Constituent
CH4
C2H5
C3Hs
CO2
N2
02
Natural Gas,%
85
10
5
Flue Gas,%
22
73.55
4.45
Given that the reactions involved are;
MgCO3 = MgO + CO2
CH4+ 202 = CO2+ 2H2O
C2H5+7h 02 = 2CO2+ 3H2O
C3Hs+ 502 = 3CO2+ 4H2O
(Atomic weights are given as Mg= 24; C = 12; H = 1;
0 = 16)
Determine the volume of air consumed in m3 per tonne of MgO produced (15 marks).
 |
4 Page 4 |
▲back to top |
Question 3
(a) Explain the reason why the blast furnace has generally been replaced by other types of
furnaces such as reverberatory and electric furnaces in the smelting of lead rich ores (5
marks).
(b) A furnace is charged with 3 000 kg/min of copper concentrate which is composed of the
following constituents;
Chalcopyrite (CuFeS2):
65%
Pyrite (FeS2):
20%
Silica (Silica):
15%
The Copper Matte produced contains
and the Slag contains
60% Cu;
15% Fe;
25%S
35% Fe
Assume that the reactions involved are;
2CuFeS2
=
Cu2S+ 2FeS + ½S2
FeS2
=
FeS+ ½S2
½S2 + 02 =
FeS+ 3h02
=
SO2
FeO + SO2
FeO + SiO2 =
FeO.SiO2
Determine the amount of;
(i) air blown into the furnace (5 marks),
(ii) matte formed (5 marks),
(iii) slag formed (5 marks), and
(iv) SO2in flue gases leaving the furnace (5 marks)
(Note: Relevant Atomic weights are Cu= 64; Fe= 56; 5 =32; Si= 28; O =16}
Question 4
(a) Discuss the factors that you would consider in order to set up a green hydrogen
manufacturing plant in a given location, and whether you consider Namibia to be a
favourable destination for such an investment. What are the global benefits of utilising green
hydrogen energy as compared to fossil fuels (5 marks).
(b) What factors would you consider to be critical before you set up an Aluminium smelter in any
country? (5 marks)
(c) Copper ore concentrate with the following assay
26.5% Cu
20%S
10% H2O
is processed to produce copper metal assaying 98.5% Cu. The off-gases are treated to
recover Sulphur in the form of Sulphuric acid. Assuming 300 tph of ore concentrate;
 |
5 Page 5 |
▲back to top |

{i) Determine the amount of H2S04 {dry) produced in tph if 85% of the S in the feed is
recovered in the H2S04acid plant (10 marks)
(ii)The process flowsheet shows that Cu is upgraded at three main stages, and 2% of the Cu
is lost at each of the stages. How much of the final product is produced in tph? (5 marks)
(Note: Atomic weights are Cu= 64, S =32, O =16, H =1}
---------------
END -------------





