 |
PCH602S - PHYSICALNCHEMISTRY -1ST OPP - NOVEMBER 2024 pdf |
 |
1 Page 1 |
▲back to top |

f
nAm I Bl A UnlVERSITY
OF SCIEnCE
FacultyofHealthN, atural
ResourceasndApplied
Sciences
Schoolof NaturalandApplied
Sciences
Departmentof Biology,
Chemistryand Physics
13JacksonKaujeuaStreet T: +264612072012
Private Bag13388
F: +264612079012
Windhoek
E: dbcp@nust.na
NAMIBIA
W: www.nust.na
QUALIFICATION: VARIOUS
QUALIFICATIONCODE:VARIOUS
COURSE:PHYSICAL CHEMISTRY
DATE: NOVEMBER 2024
DURATION: 3 HOURS
LEVEL:6
COURSECODE: PCH602S
SESSION:1
MARKS: 100
FIRST OPPORTUNITY: EXAMINATION QUESTION PAPER
EXAMINER:
MODERATOR:
Prof Habauka Kwaambwa
Dr Euodia Hess
INSTRUCTIONS
1. Answer ALL the questions in Sections A and B.
2. Answer all questions on the separate answer sheet.
3. Please write neatly and legibly.
4. Do not use the left side margin of the exam paper. This must be allowed for the
examiner.
5. No books, notes and other additional aids are allowed.
6. Mark all answers clearly with their respective question numbers.
PERMISSIBLE MATERIALS
Non-Programmable Calculator
ATTACHMENTS
List of Useful Constants and Equation
THIS QUESTION PAPER CONSISTS OF 8 PAGES (Including this front page and a list of useful
constants and equation as an attachment)
 |
2 Page 2 |
▲back to top |

SECTION A: MULTIPLE CHOICE QUESTIONS
[20]
There are 10 questions in this section. Choose the correct answer. Each question carries 2
marks.
1. A gas sample at 25°C occupies 105 ml at a pressure of 698 Torr. What volume will it
occupy at this temperature and a pressure of 1.91 atm?
A. 50.5 ml
B. 3.84 l
C. 59.9 ml
D. 0.0771 l
E. None of the above
2. A system suffers an increase in internal energy of 80 J and at the same time has 50 J of
work is done on it. What is the heat change of the system?
A. + 130 J
B. + 30 J
C. -103 J
D. -30 J
E. 0 J
3. The llH 0 for the following reaction at 298 K is - 36.4 kJ.
1
1
-H
2
2 (g)
+-Br
2
2 (e)
HBr(g)
Calculate llU 0 at 298 K.
A. -35.2 kJ
B. + 35.2 kJ
C. - 36.4 kJ
D. -37.6 kJ
E. + 37.6 kJ
4. The entropy will usually increase when:
I.
Molecule is broken into two or more smaller molecules.
II.
A reaction occurs that results in an increase in the number of moles of gas.
Ill. A solid changes to a liquid.
IV. A liquid change to a gas
A. I only
B. II only
C. Ill only
D. IV only
E. I, II, 111a, nd IV
Physical Chemistry (PCH602S}
1st Opportunity November 2024
2
 |
3 Page 3 |
▲back to top |

5. For which of the following reactions would ~H 0 for the reaction be equal to
of formation):
A. PC'3(g) + -1 O2(g)
2
POCl3(g)
1
1
B. - N2O(g) + - O2(g)
2
4
NO(g)
C. CaO(s) + SO2(g) CaSO3(s)
D. 2N2(g) + O2(g) 2N2O(g)
E. Al(s) + -3 H2(g) + -3 O2(g)
2
2
Al(OH)3(s)
(heat
6. Which of the following aqueous solutions has the highest ionic strength (all salts are
fully soluble).
A. 1 M NaCl
B. 1 M Ca(NO3)z
C. 1 M CuSO4
D. 1 M Al2(SO4)3
E. 1 M Na3PO4
7. A mixture of aqueous Na3PO4 and ZnSO4 (both salts fully soluble) has a certain ionic
strength, x. Which ion has the highest activity coefficient according to the Debye-
Huckel Limiting Law?
A. Na+
B. SO~-
C. Zn2+
D. PO!-
E. Insufficient information
8. The following reaction occurs in an electrochemical cell:
3Cu2+ + 2Cr 2Cr3+ + 3Cu
The E0 for the cell is
A. 0.40 V
B. 1.08 V
C. 0.75 V
D. 2.50 V
E. Insufficient information
Given:
E0 ( Cu2+ /Cu)= 0.337V
E0 ( Cr3+ /Cr) =-0.74V
Physical Chemistry (PCH602S)
1st opportunity November 2024
3
 |
4 Page 4 |
▲back to top |
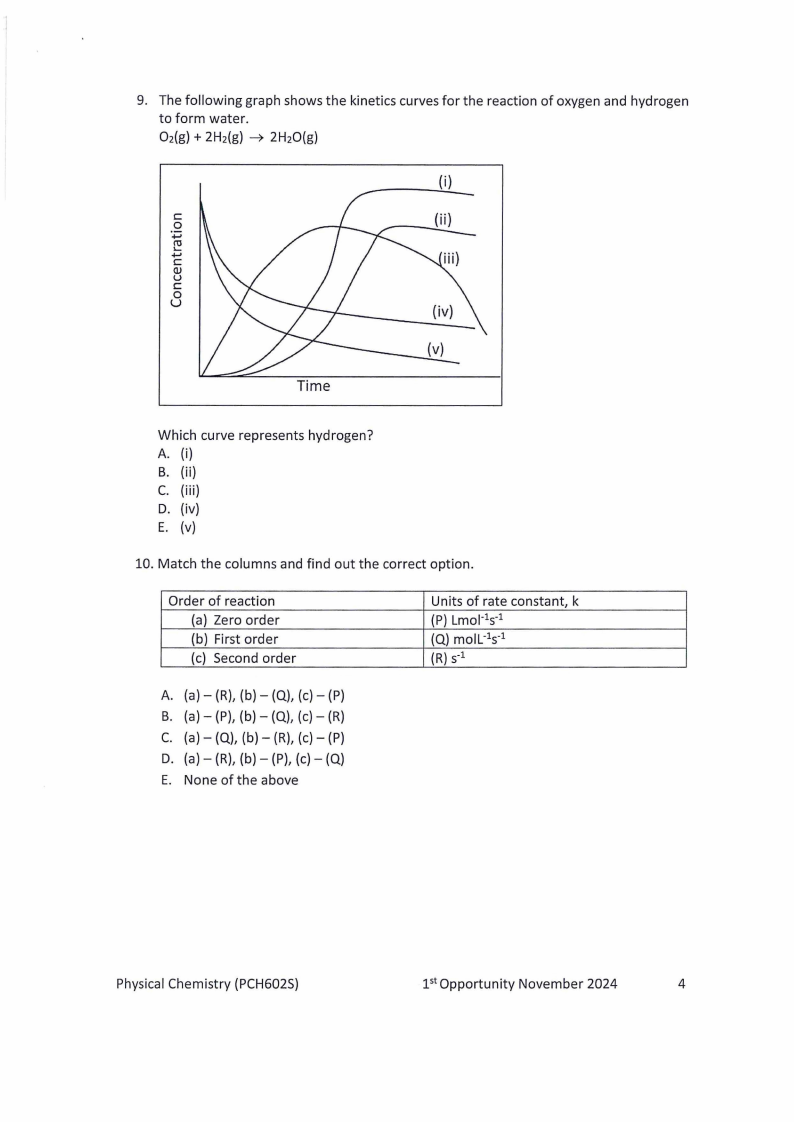
9. The following graph shows the kinetics curves for the reaction of oxygen and hydrogen
to form water.
O2(g)+ 2H2(g) 2H2O(g)
( i)
C:
0
+-'
+-'
C:
Qu J
C:
u0
Time
Which curve represents hydrogen?
A. (i)
B. (ii)
C. (iii)
D. (iv)
E. (v)
10. Match the columns and find out the correct option.
Order of reaction
(a) Zero order
(b) First order
(c) Second order
Units of rate constant, k
(P) Lmo11-s-1
(Q) mo1L-1s-1
(R) s-1
A. (a)- (R), (b)- (Q), (c)- (P)
B. (a) - (P), (b) - (Q), (c) - (R)
C. (a) - (Q), (b) - (R), (c) - (P)
D. (a) - (R), (b) - (P), (c) - (Q)
E. None of the above
Physical Chemistry (PCH602S)
pt Opportunity November 2024
4
 |
5 Page 5 |
▲back to top |

SECTIONB
[80]
There are THREEEquestions in this section. Answer all Questions.
QUESTION 1
[31]
(a) Using Redlich-Kwong equation state for a non-ideal gas, answer the following
questions:
P = - RT _ I a
V-b T2v(v+b)
(i) What do the terms a and b relate to?
(2)
(ii) State the condition and show that the equation reduces to an ideal gas equation
of state.
(4)
(iii) State under what conditions is positive deviation and negative deviation from
ideal behaviour of gases observed?
(2)
(b) State whether q, w, LiU and LiH are positive, negative or zero for adiabatic
compression of an ideal gas.
(4)
(c) One mole of an ideal gas at 10 bar and 298 K is expanded reversibly isothermally to
final pressure of 1 bar. Calculate q, w, ~U and ~H for the gas.
(7)
(d) The following reactions are being proposed for the green hydrogen production and
use in Namibia. For each of the following, deduce if LiH = LiU, LiH < LiU or LiH > LiU,
and the entropy change, .6.S,is greater than zero, less than zero or zero.
(4)
(i)
2H2O(I) O2(g)+ 2H2(g)
Green hydrogen production by electrolysis of seawater after desalination
(splitting of water)
(ii) 3H2 (g) + N2(g) 2NH3(g)
Production of green hydrogen carrier for safer storage and transportation
to the European market
(iii) 2NH3(g)+ H2SO4(aq) (NH4)i$Q4(I)
Production of fertiliser, which is then crystallised and dried to produce the final
product
(iv) Fe3Q4(S+) 4H2(g) 3Fe(s)+ 4H2O(I)
One of the reactions in steel production
(e) Usethe data below to calculate LiH 0 , LiS0 , LiGO and Kpfor the reaction at 298K
CH3CH2CH2CH(3g) ='7CH3CH(CH3}z(g)
Butane
2-Methylpropane
Compound
Butane
2-Methylpropane
LlH1 I kJmol- 1
-126 kJmo1-1
-125 kJmo1-1
S0 (298K)
270 JK-1mo1-1
295 JK-1 mo1-1
(8)
Physical Chemistry (PCH602S)
1st Opportunity November 2024
5
 |
6 Page 6 |
▲back to top |

QUESTION 2
[29]
(a) Give the equations and SI units for the terms electrolytic conductivity, K, and molar
conductivity, Ax,
(2)
(b) Explain briefly the difference between a strong and weak electrolyte and show
schematically on the same plot how the molar conductivity of a typical example of each
varies with concentration.
(4)
(c) Compare and contrast the conductometric titration graphs of (i) a strong acid with a
strong base; (ii) a weak acid with a strong base; and (iii) mixture of a strong acid and weak
acid with a strong base.
(4)
(d) The limiting molar conductivities (in Sm2moI-1) of sodium chloride, sodium formate
(HCOONa} and hydrochloric acid are 1.264 x 10-2, 1.046 x 10-2 and 4.261 x 10-2,
respectively. The conductivity of 0.0100 moldm-3 formic acid (HCOOH} at 25°C is
5.07 x 10-2sm-1. Calculate the dissociation constant of 0.01 moldm- 3formic acid. (6)
(e) Use the Debye-Hi.ickel limiting law for the mean activity coefficient of an electrolyte:
log 10y± = -Alz+z-lfi
to calculate the mean activity coefficient for 0.010 molkg- 1 CuCl2 in:
(i) water, and;
(ii) 0.010 molkg-1 NaCl, given that A= 0.509 mol-½ kg½.
(6)
(f) Two platinum electrodes were immersed in aqueous solution of a nitrate M(NO3)2,where
M is a metal which forms a divalent ion. After passing a current of 0.501 amps for 63
minutes, 1.10 g of the metal M had been deposited on the platinum cathode. Using
Faradays laws of electrolysis, calculate the molar mass of the metal M.
(3)
(g) In the cell
I I I I Pt Fe2+, Fe3+ Hg~+ Hg
the standard emf of the half-cells are:
E0 (Fe3+, Fe2+)= +0.771 V and E0 (Hg~+I Hg}= +0.789 V.
Deduce the overall reaction of cell. Will the reaction occur spontaneously as written in the
cell?
(4)
QUESTION 3
[20]
(a) Nitrogen pentoxide (N2Os}gas decomposes according to the reaction
2N2Os 4NO2 + 02
At 328K, the rate of the reaction under certain conditions is 0.75 x 10-4 mol L-1s-1.
Assuming that none of the intermediates have appreciable concentrations, calculate
the values of the following:
(6)
(i)
dlN20 5 J -
(ii)
Physical Chemistry (PCH602S)
1st Opportunity November 2024
6
 |
7 Page 7 |
▲back to top |
(iii)
dt
(b) Consider a reaction A
P. The integrated rate law for the reaction is:
_1 __ l_=kt
[A] [A0]
(i)
State the two reaction requirements needed in order to derive the equation
above.
(1)
(ii) What is the order of the reaction? What are the units of the rate constant if
the rate is in mol L-1 min-1?
(2)
(iii) What plot would you construct the plot to determine the rate constant, k, for
the reaction? Label the axes on diagram and sketch the graph that you would
expect. How would you get k from the graph?
(2)
(iv) Derive the half-life expression for this reaction.
(3)
(c) The table below gives experimental data for the half-lives, t0_5 , of different reactions
as a function of the initial reactant concentration, Co, Determine the order of each of
the three reactions.
(6)
to.sf min
Co/ moldm- 3
Reaction 1 Reaction 2 Reaction 3
4
5
120
100
2
5
60
200
---------------------------------------------------------------------------------------------------------------------------
END OF EXAMINATION QUESTION PAPER
Physical Chemistry {PCH602S)
1st Opportunity November 2024
7
 |
8 Page 8 |
▲back to top |

LIST OF USEFUL EQUATION AND CONSTANTS
Van der Waals eqn. p = nRT _ n 2 a = _R_T__ a_
V - nb V 2 V-b
2
V
Universal Gas constant
R
=
8.314 J K-1 moI-1
Boltzmann's constant,
k
=
1.381 X 10-23 J K-1
Planck's constant
h
=
6.626 X 10-34 J S
Debye-Huckel's constant, A
=
0.509 (mol dm-3) 112 or mol-0·5kg05
Faraday's constant
F
=
96485 C moI-1
Mass of electron
Velocity of light
me =
C
=
9.109 X 10-31 kg
2.998 x 108 m s-1
Avogadro's constant
1 electron volt (eV)
NA =
=
6.022 X 1023
1.602 X 10-19 J
1 atm = 101325 Pa= 760 mm Hg= 760 torr; 1 bar= 105 Pa
Physical Chemistry (PCH602S)
l51 Opportunity November 2024
8





