 |
ICH602S - INORGANIC CHEMISTRY - 1ST OPP - NOV 2022 |
 |
1 Page 1 |
▲back to top |

nAm I BIA un IVE RS ITY
OF SCIEn CE Ano TECHn OLOGY
FACULTYOF HEALTH,NATURALRESOURCESAND APPLIEDSCIENCES
DEPARTMENT OF NATURALAND APPLIEDSCIENCES
QUALIFICATION: BACHELOROF SCIENCE
QUALIFICATION CODE: 07BOSC
LEVEL:6
COURSECODE: ICH602S
COURSENAME: INORGANIC CHEMISTRY
SESSION:NOVEMBER 2022
PAPER:THEORY
DURATION: 3 HOURS
MARKS: 100
FIRSTOPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S) DR. EUODIA HESS
MODERATOR: PROF HABAUKA KWAAMBWA
INSTRUCTIONS
1. Answer ALL the questions.
2. Write clearly and neatly.
3. Number the answers clearly
4. All written work must be done in blue or black ink and sketches can
be done in pencil
5. No books, notes and other additional aids are allowed
PERMISSABLEMATERIALS
Non-programmable calculators
ATTACHMENTS
1. List of useful constants
2. Periodic Table
THIS QUESTION PAPERCONSISTSOF 8 PAGES
(Including this front page, list of constants and periodic table)
 |
2 Page 2 |
▲back to top |

SECTIONA
(40]
QUESTION 1: Multiple Choice Questions
[40]
• Thereare 20 multiple choicequestions in this section. Eachquestion carries
2 marks.
• Answer ALLquestions by selecting the letter of the correctanswer.
• Choosethe best possibleanswerfor each question, even if you think there is
another possible answer that is not given.
1. Which of the following ranks regions of the electromagnetic spectrum in proper order from
highest to lowest frequency.
A. radio> x-rays> gamma rays> visible> microwaves
B. gamma rays> x-rays >visible> microwaves> radio
C. microwaves> gamma rays> x-rays >visible> radio
D. x-rays > gamma rays> microwaves >visible> radio
2. Which of the following regions of the electromagnetic spectrum has the lowest frequency?
A. x-ray
B. gamma ray
C. ultraviolet
D. infrared
3. A device emits light at 244.4 nm. What is the frequency of this radiation?
A. 1.23 x 1015 Hz
B. 8.14 x 10-37 Hz
C. 8.14 x 10-19 Hz
D. 3.69 x 1026 Hz
4. What is the wavelength of a photon that has an energy of 3.097 x 104 J?
A. 3.1 x 1013 nm
B. 6.42 x 10-21 nm
C. 9.29 x 1021 nm
D. 6.16 x 1012 nm
5. What is the wavelength of light emitted when the electron in a hydrogen atom undergoes a
transition from level n = 8 to level n = 2?
A. 1.7 x 10-27 kJ/mol
B. 2.57 x 106 kJ/mol
C. 5.11 x 10-19 kJ/mol
D. 3.89 x 10-7 kJ/mol
6. What is the hybridization of the central atom in a molecule with a tetrahedral molecular
geometry?
A. sp2
B. sp
C. sp3
D. sp3d
Page 2 of 8
 |
3 Page 3 |
▲back to top |

7. What is the hybridization of each carbon atom in benzene, C5H5?
A. sp
B. sp2
C. sp3
D. sp4
8. For which of the following molecules does the carbon atom have sp3 hybridization?
A. Cl2CO
B. CO
C. CS2
D. CH2Cb
9. What is the molecular geometry around a central atom that is sp 2 hybridized, has three sigma
bonds, and one pi bond?
A. trigonal-planar
B. trigonal-pyramidal
C. square planar
D. T-shaped
10. Which of the following concerning a and rebonds is/are correct?
A. Pi bonds are formed from unhybridized p orbitals
B. Both A and D
C. Sigma bonds may only be formed from unhybridized orbitals Pi bonds are formed from
unhybridized p orbitals
D. A pi bond has an electron distribution above and below the bond axis
11. A molecular orbital that decreases the electron density between two nuclei is said to be __ .
A. Hybridized
B. Bonding
C. pi-bonding
D. antibonding
12. The following valence molecular orbital energy level diagram is appropriate for which one of the
listed species?
0*2p
n*2p 1'
1'
02p
1'-J;
1'-J;
TI2p
1'-J;
0*2s
1'-J;
02s
1'-J;
A. Ff+
B. cf+
C. Brf+
D. Nf+
Page 3 of 8
 |
4 Page 4 |
▲back to top |
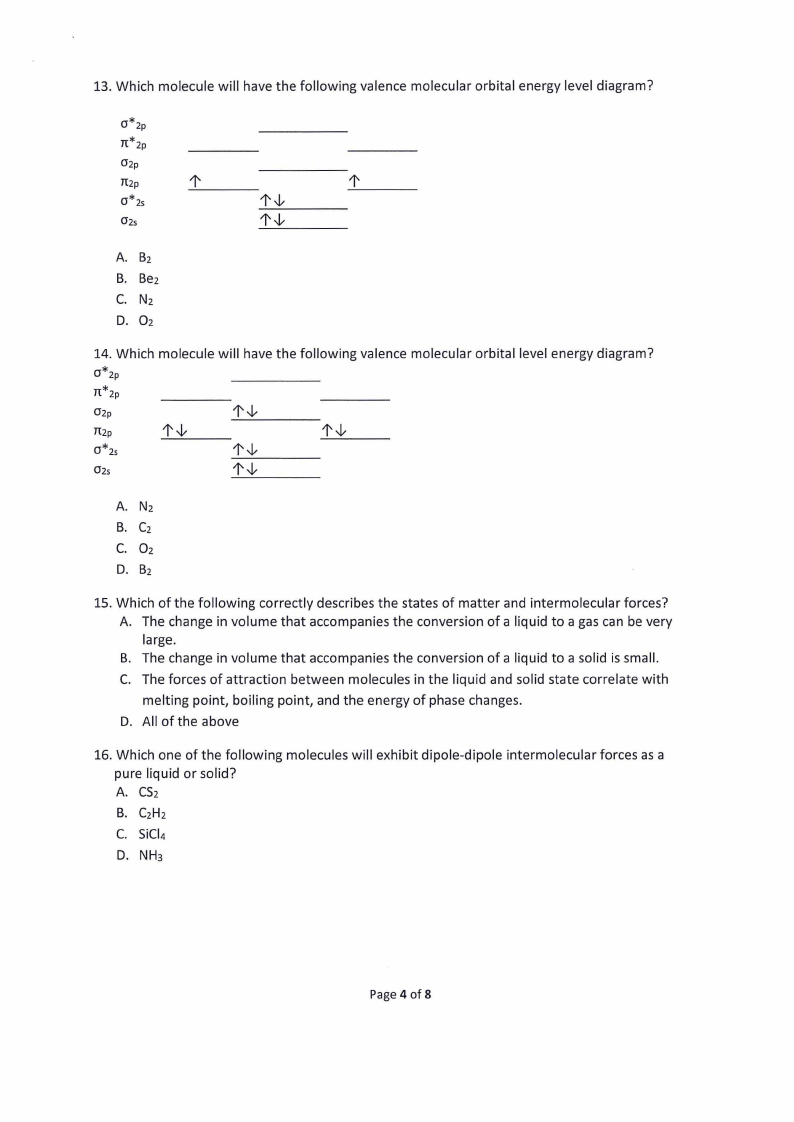
13. Which molecule will have the following valence molecular orbital energy level diagram?
0*2p
n*2p
02p
TI2p
0*2s
1' -1,
02s
1' -1,
A. B2
B. Be2
C. N2
D. 02
14. Which molecule will have the following valence molecular orbital level energy diagram?
0*2p
n*2p
02p
1' -1,
TI2p
1' -1,
0*2s
1'-1,
02s
1' -1,
1' -1,
A. N2
B. C2
C. 02
D. B2
15. Which of the following correctly describes the states of matter and intermolecular forces?
A. The change in volume that accompanies the conversion of a liquid to a gas can be very
large.
B. The change in volume that accompanies the conversion of a liquid to a solid is small.
C. The forces of attraction between molecules in the liquid and solid state correlate with
melting point, boiling point, and the energy of phase changes.
D. All of the above
16. Which one of the following molecules will exhibit dipole-dipole intermolecular forces as a
pure liquid or solid?
A. CS2
B. C2H2
C. SiCl4
D. NH3
Page4of8
 |
5 Page 5 |
▲back to top |

17. Which of the following bonds can potentially contribute to the formation of a hydrogen bond
in a solid or liquid?
A. Ge-H
B. Si-H
C. 1-H
D. N-H
18. Hydrogen bonding is present in all of the following molecular solids EXCEPT__ .
A. H2SO4
B. CH3OH
C. HF
D. CH3OCH3
19. As pure molecular solids, which of the following exhibit only induced dipole/induced
forces: CO2,CH2Cl2,and SO2?
A. CO2only
B. CH2Clzonly
C. CO2and CH2Cl2
D. SO2only
dipole
20. What intermolecular force or bond is primarily responsible for the solubility of carbon
monoxide (CO) in water?
A. dipole/induced dipole force
B. dipole-dipole force
C. hydrogen bonding
D. ion-induced dipole force
SECTIONB:
[60]
There are THREE questions in this section. Answer all questions. Show clearly, where necessary,
how you arrive at the answer as all working will carry marks.
Question 1
[22)
1.1 Name the species and give the valence electron counts to the metal atoms in:
(10)
a) [Fe(CO)s]
b) [Mn2(COho
c) [V(CO)5]
d) [Fe(CO)4J2-
e) Rh(Me)(CO)z(PPh3)]
1.2 What hapticities are possible for the interaction
d-block metal atom such as cobalt?
a) C2H4
b) cyclopentadienyl
c) C5H5
d) cyclooctadiene
e) cyclooctatetraene
of each of the following
ligands with a single
(8)
Page 5 of 8
 |
6 Page 6 |
▲back to top |

1.3 Give the electron count of:
(4)
a) [Ni(ri3-C3Hshl
b) [Co(ri3-C3Hs)(C0)2]
Question 2
[30]
2.1 Decide which type of intermolecular forces is involved in:
(6)
a) 02
b) CH30H
c) N2 in H20
2.2 The molar enthalpy of vaporization of methanol is 35.2 kJ/mol at 64.6 °c.How much energy
Is required to evaporate 1.00 kg of methanol at 64.6 °C?
(3)
2.3 Gold has a face centered unit cell and it's density is 19.32 g/cm 3. Calculate the radius of gold
atom.
(10)
2.4 Iron has a density of 7.8740 g/cm 3and the radius of an iron atom is 126 pm. Verify that solid
iron has a body-centered cubic unit cell.
(10)
2.5 A soft waxy solid melts over a temperature range from 120 °cto 130 °c.It doesn't dissolve
in water and does not conduct electricity. These properties are consistent with its identity
as a ___
solid.
(1)
Question 3
[8]
Define Hard and Soft acids and bases (HSAB)theory. How would you characterize hard acids
and bases?
THEEND
GOODLUCK
Page 6 of 8
 |
7 Page 7 |
▲back to top |

USEFULCONSTANTS:
Gas constant, R = 8.3145 J · mo1-1 · K-1 = 0.083145 dm 3 ·bar· mo1-1 · K-1 = 0.08206 L atm mo1-1 · K-1
1 Pa · m3 = 1 kPa.L = 1 N · m = 1 J
1 atm = 101 325 Pa= 760 mm Hg= 760 torr
Avogadro's Number, NA= 6.022 x 1023 mo1-1
Planck's constant, h = 6.626 x 10-34 Js
Rydberg constant, Rh= 2.18 x 10-18 J
Speed of light, c = 2.998 x 108 ms-1
Page 7 of 8
 |
8 Page 8 |
▲back to top |
PERIODIC TABLE OF THE ELEMENTS
18
l
2
H
1.00794 2
13
14
15
He
16
17 4.00260
34
56
7
8 9 IO
Li Be
B C N 0 F Ne
6.941 9.01218
10.81 12.011 14.0067 15.9994 18.9984 20.179
11 12
Na Mg
22.9898 24.305 3
4
5
6
13 14 15 16 17 18
Al Si p s Cl Ar
7
8
9
10
11
12 26.9815 28.0855 30.9738 32.06 35.453 39.948
19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
39.0983 40.08 44.9559 47.88 50.9415 51.996 54.9380 55.847 58.9332 58.69 63.546 6538 69.72 72.59 74.9216 78.96 79.904 83.8
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
-,:,
Rb Sr y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Q.)
Q'Q
Cl)
85.4678 87.62 88.9059 91.22 92.9064 95.94 (98) 101.07 102.906 106.42 107.868 112.41 114.82 118.69 121.75 127.6 126.9 131.29
00
55 56 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
0
-t,
00
Cs Ba Lu Hf Ta w Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
132.905 137.33 174.967 178.49 180.948 183.85 186107 190.2 192.22 195.08 196.967 200.59 204383 207.2 208.908 (209) (210) (222)
87 88 103 104 105 106 107 108 109 110 111 112
114
ll(i
118
Fr Ra Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub
Uuq
(223) 226.025 (260) (261) (262) (263) (264) 1265) (268) (269) (272) (269)
Uuh
Uuo
L11ntb11oides: 57 58 59 60 61 62 63 64 65 66 67 68 69 70
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
138.906 140.12 140.908 144.24 (145) 150.36 151.96 157.25 158.925 162.50 161.930 167.26 166.934 173.04
Actinides:
89 90 91 92 93 94 95 96 97 98 99 100 101 102
Ac Th Pa u Np Pu Am Cm Bk Cf Es Fm Md No
227.028 232.038 231.036 238.029 237.048 (244) (243) (247) (247) (251) (252) (257) (258) (259)





