 |
SSP701S - SOLID STATE PHYSICS - 1ST OPP - JUNE 2023 |
 |
1 Page 1 |
▲back to top |

nAmlBIA UnlVERSITY
OF SCIEnCE Ano TECHnOLOGY
FACULTYOF HEALTH,NATURAL RESOURCESAND APPLIEDSCIENCES
SCHOOL OF NATURAL AND APPLIED SCIENCES
DEPARTMENT OF BIOLOGY, CHEMISTRY AND PHYSICS
QUALIFICATION: BACHELOROF SCIENCE
QUALIFICATION CODE: 07BOSC
LEVEL: 7
COURSE CODE: SSP701S
COURSE NAME: SOLIDSTATEPHYSICS
SESSION: JUNE 2023
DURATION: 3 HOURS
PAPER: THEORY
MARKS: 100
FIRSTOPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER{S) Prof Dipti R. Sahu
MODERATOR: Dr Zivayi Chiguvare
INSTRUCTIONS
1. Answer all five questions.
2. Write clearly and neatly.
3. Number the answers clearly.
PERMISSIBLEMATERIALS
Non-programmable Calculators
THIS QUESTION PAPER CONSISTS OF 3 PAGES (Including this front page}
 |
2 Page 2 |
▲back to top |
Question 1
[20]
1.1 a.-Cohas an hep structure with lattice spacings of a=2.51 Aand c = 4.07 A,~-Co isfee, with cubic
lattice spacing of 3.55 A.What is the difference in density between the two forms?
(4)
1.2 Name various types of cubic class of crystalline structures. Explain their characteristics. Give the
values of parameters which distinguish them from each other.
(6)
1.3 (a) Calcium has a face-centered cubic structure with an ionic radius of 1.06 A.Calculate the
interplanar separation for (111) planes.
(5)
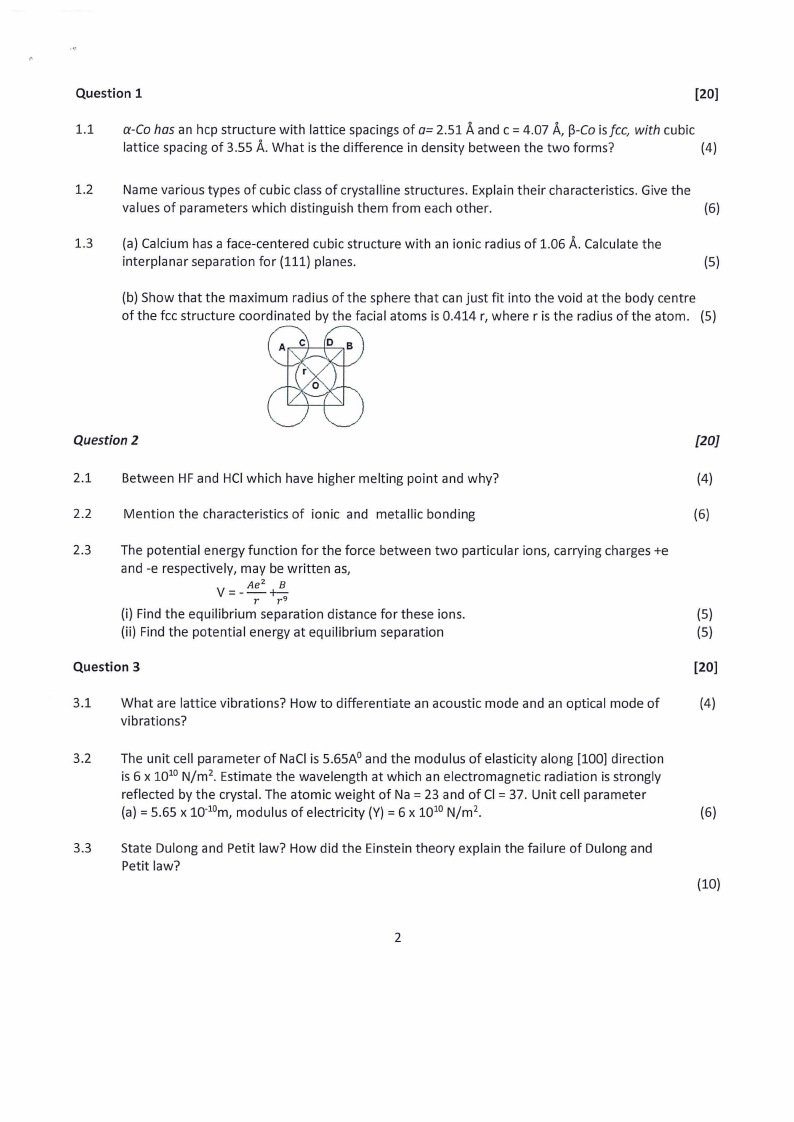
(b) Show that the maximum radius of the sphere that can just fit into the void at the body centre
of the fee structure coordinated by the facial atoms is 0.414 r, where r is the radius of the atom. (5)
Question 2
[20]
2.1 Between HFand HCIwhich have higher melting point and why?
(4)
2.2 Mention the characteristics of ionic and metallic bonding
(6)
2.3 The potential energy function for the force between two particular ions, carrying charges +e
and -e respectively, may be written as,
V=--+A-e 2
r
B
r9
(i) Find the equilibrium separation distance for these ions.
(5)
(ii) Find the potential energy at equilibrium separation
(5)
Question 3
[20]
3.1 What are lattice vibrations? How to differentiate an acoustic mode and an optical mode of
(4)
vibrations?
3.2 The unit cell parameter of NaCl is 5.65A0 and the modulus of elasticity along [100] direction
is 6 x 1010 N/m 2. Estimate the wavelength at which an electromagnetic radiation is strongly
reflected by the crystal. The atomic weight of Na= 23 and of Cl= 37. Unit cell parameter
(a) = 5.65 x 10-10m, modulus of electricity (Y) = 6 x 1010 N/m 2.
(6)
3.3 State Dulong and Petit law? How did the Einstein theory explain the failure of Dulong and
Petit law?
(10)
2
 |
3 Page 3 |
▲back to top |

Question 4
[20]
4.1 What is classical free electron theory? Write the significance of the free electron theory.
(4)
4.2 The conductivity of silver is 6.5 x 107 per Ohm perm and number of conduction electrons per
m3 is 6 x 1028 • Find the mobility of conduction electrons and the drift velocity in an electric
field of 1 V/m. Given m = 9.1 x 10-31 kg and e = 1.602 x 10-19 C.
(6)
4.3 Derive Wiedemann-Franz law? Mention which factors are affecting the Wiedemann Franz law? (10)
Question 5
[20]
5.1 What is density of energy states? Draw a plot between density of states and the energy of
electrons
(4)
5.2 In a semiconductor the effective mass of an electron is 0.07mo and that of a hole is 0.4mo,
where m0 is the free electron mass. Assuming that the average relaxation time for the holes
is half that for the electrons, calculate the mobility of the holes when the mobility of the
electrons is 0.8m 2 volt- 1 sec-1.
(6)
5.3
What is direct and indirect band gap semiconductors, explain with diagram
(10)
Given fundamental constants.
Speed of light = 3x108m/s
Planck constant= 6.626 x10-34 Js
Mass of electron= 9.1 x 10-31 kg
Charge of electron= 1. 6 x 10-19 C
Avogadro number= 6.022 x 1023 /mole
Boltzmann Constant =1.38 x 10-23 JK-1
3





