|
AOC811S - ADVANCED ORGANIC CHEMISTRY - 2ND OPP - JULY 2022 |
 |
1 Page 1 |
▲back to top |

NAMIBIA UNIVERSITY
OF SCIENCE AND TECHNOLOGY
FACULTY OF HEALTH, APPLIED SCIENCES AND NATURAL RESOURCES
DEPARTMENT OF NATURAL AND APPLIED SCIENCES
QUALIFICATION: BACHELOR OF SCIENCE HONOURS
QUALIFICATION CODE: O8BOSH
LEVEL: 8
COURSE CODE: AOC811S
COURSE NAME: ADVANCED ORGANIC CHEMISTRY
SESSION: JULY 2022
PAPER: THEORY
DURATION: 3 HOURS
TOTAL MARKS: 100
SUPPLEMENTARY / SECOND OPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S) | DR. MARIUS MUTORWA
MObERATOR; | DR. RENATE HANS
INSTRUCTIONS
Answer ALL the questions.
Write clearly and neatly.
Number the answers clearly
All written work must be done in blue or black ink and sketches can
be done in pencil
5. No books, notes and other additional aids are allowed
PERMISSIBLE MATERIALS
Non-programmable Calculators
ATTACHMENTS
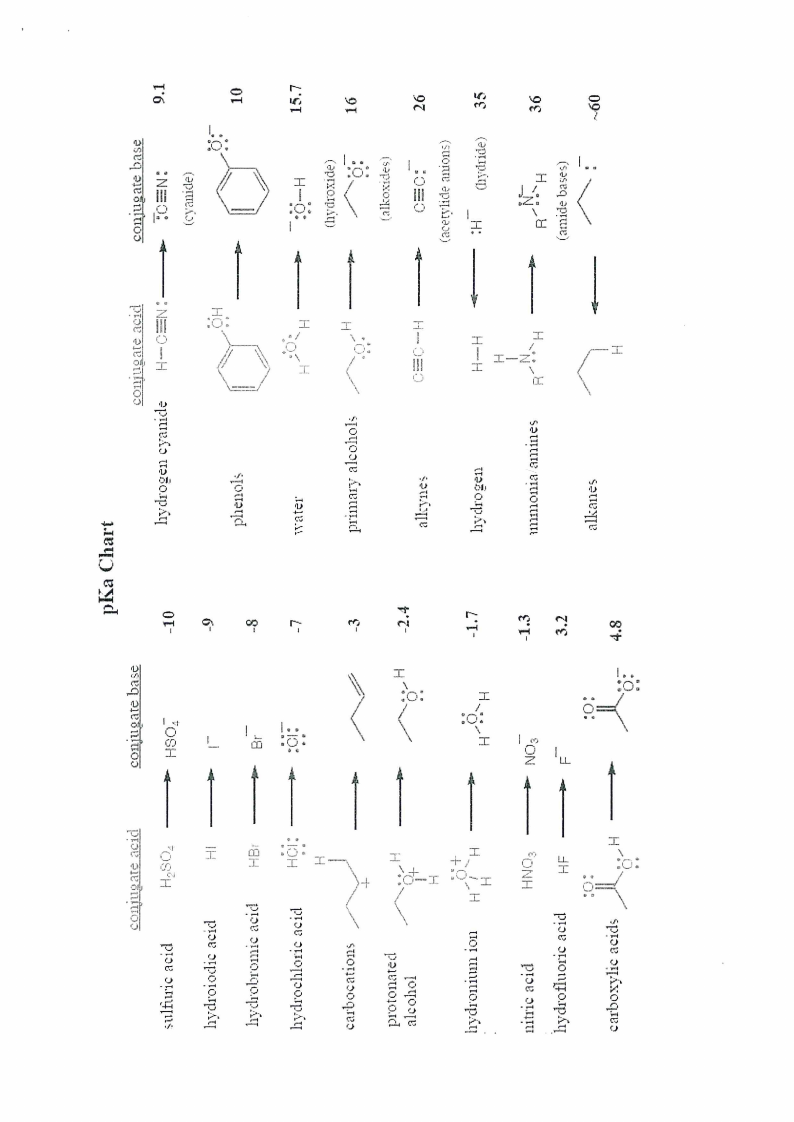
pKa Chart and Periodic Table
THIS QUESTION PAPER CONSISTS OF 8 PAGES
(Including this front page and attachments)
 |
2 Page 2 |
▲back to top |
QUESTION 1:
[20]
Question type: Enolates and Carbon Nucleophiles
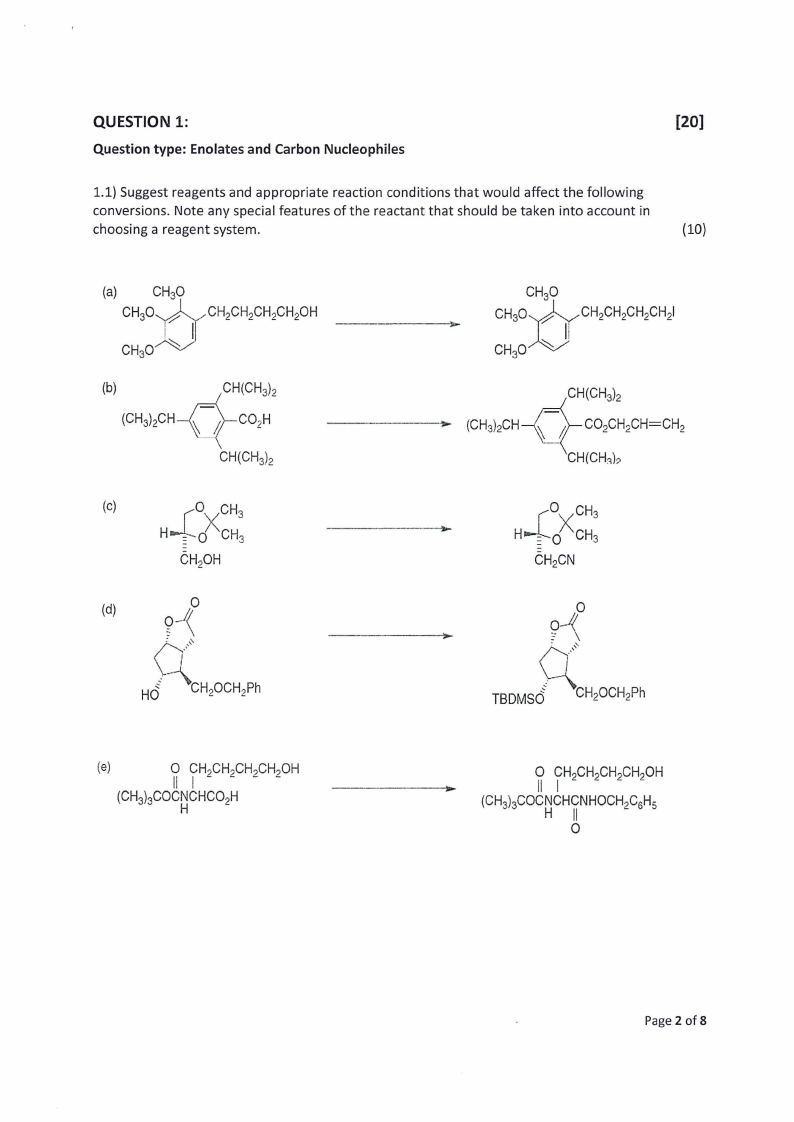
1.1) Suggest reagents and appropriate reaction conditions that would affect the following
conversions. Note any special features of the reactant that should be taken into account in
choosing a reagent system.
(10)
(a) CHO CH730 -CHCHECHECHLOR
-
CH,O7 S~
(b)
CH(CHs)>
(CHs),CH—)x—CO;H
CH(CHs)5
—_-—
CH,O
. maeseouencnen
CH,0~ S |
CH(CHs).
> (CHs)CH—{.7_:)--CO2CH,CH=CH,
CH(CHa)>
(c)
wx
Hei~0 ‘CHs
CH,OH
(d)
Ct9i. -4#0
HG -CH2OCH Ph
I
——z
SOs
wg CH;
CHCN
Co.lk -
TBDMSG -CH2OCHPh
(e)
i CHoCH;CH.CH,OH
(CHs},COCNCHCO,H
-_-----o
7 CH.CH;CH,CH,OH
(CH)s;COCNCHCNHOCH,C,H,
H 0|
Page 2 of 8
 |
3 Page 3 |
▲back to top |
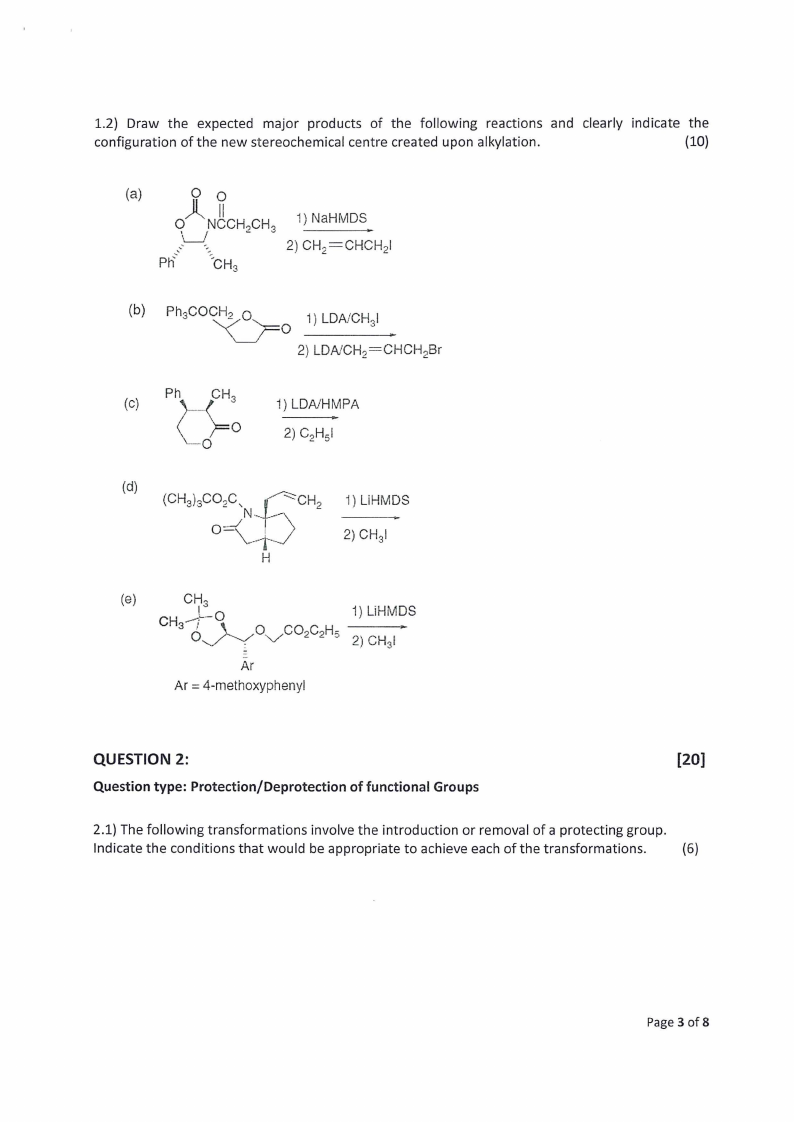
1.2) Draw the expected major products of the following reactions and clearly indicate the
configuration of the new stereochemical centre created upon alkylation.
(10)
°
iO° NCCH,CH,
1) NAaHMDS
oo
Ph
CH,
2) CH,=CHCHp|
(b) =Ph3COCH2 6
1) LDA/CHs|
2) LDA/CH,=CHCH,Br
(Cc)
\\.
(JRO psaanl O
1) LDA/HMPA
Caz s
(d) (CH,),COo.wC.
CH, 1) LIHMDS
Vp
2) CHa!
H
(2)
Cre
CH;"9
OY
O. Ver ,COe:eCeHs=
1) LI. HMDS
2) CHal
Ar
Ar = 4-methoxyphenyl
QUESTION 2:
[20]
Question type: Protection/Deprotection of functional Groups
2.1) The following transformations involve the introduction or removal of a protecting group.
Indicate the conditions that would be appropriate to achieve each of the transformations.
(6)
Page 3 of8
 |
4 Page 4 |
▲back to top |
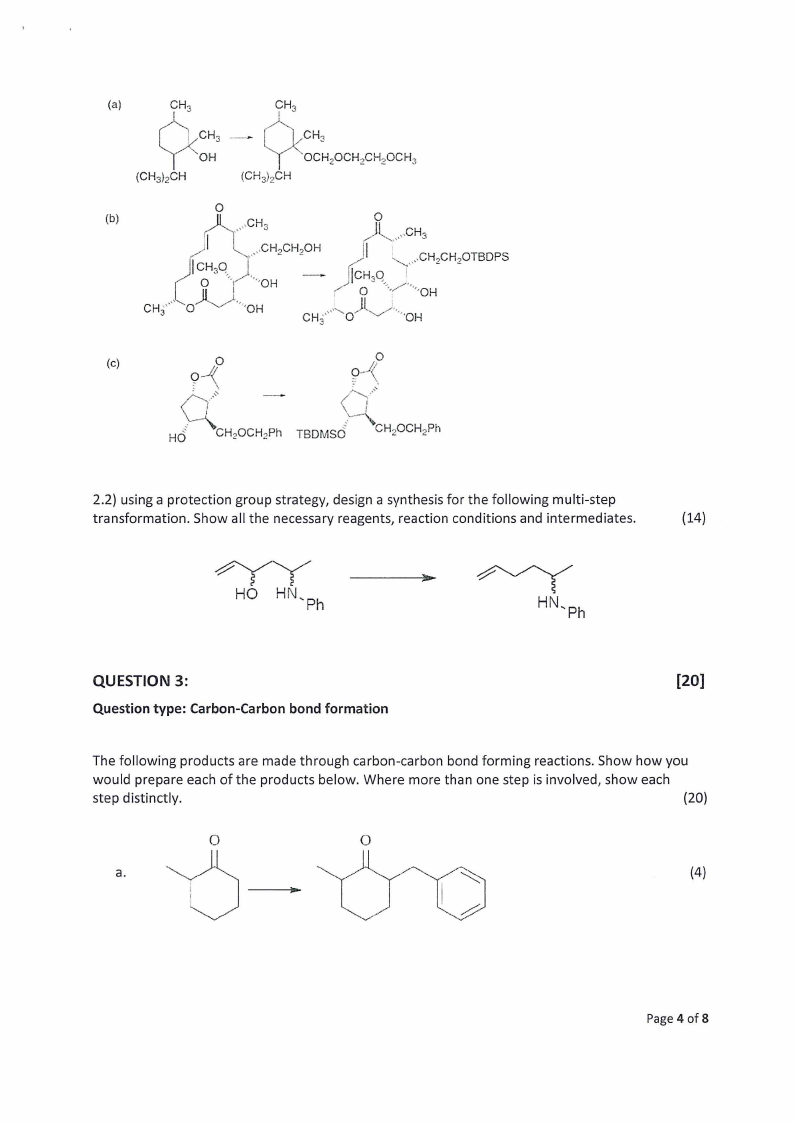
(a)
CH,
a{ CH;
N OH
(CH3)2CH
CH,
> a | CH,
{ co. “OCH,OCH.CH,OCH,
(CHg)oCH
(b)
aNi CHs 3
UAoL .CHs
|.a“
\\_; CHCHOH
|Co H;Qgy|
(|
~
»CH.CH,OTBDPS
Jf|ioCH50, ye
CH”
\\
oO
SNA
‘OH
“Qe Oy
o
Ih
An
(c)
Oo
fy Oo
oX
ok
.»
oe
Z ~=
C
|
4
ud : NH OCH:Ph TapMsG = = > CH2OCH,Ph
2.2) using a protection group strategy, design a synthesis for the following multi-step
transformation. Show all the necessary reagents, reaction conditions and intermediates.
(14)
De
HO HN, Ph
aN
ANS oy,
QUESTION 3:
[20]
Question type: Carbon-Carbon bond formation
babe The following products are made through carbon-carbon bond forming reactions. Show how you
would prepare each of the products below. Where more than one step is involved, show each
step distinctly.
(20)
Page 4 of 8
 |
5 Page 5 |
▲back to top |
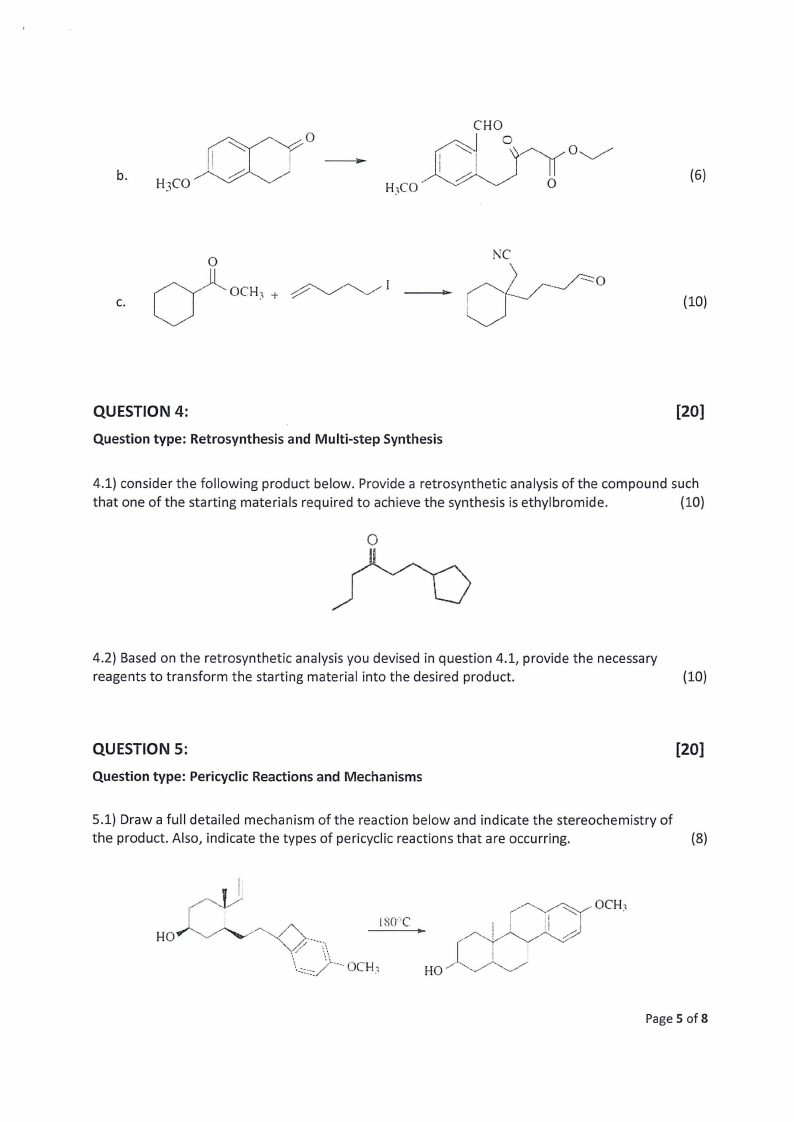
°
|>
~
cy
OV
b.
H3CO
LA ~
H3CO” ; ZA
O
(6) 6
"OC 2 ose! Ce
—=O
(10)
QUESTION 4:
[20]
Question type: Retrosynthesis and Multi-step Synthesis
4.1) consider the following product below. Provide a retrosynthetic analysis of the compound such
that one of the starting materials required to achieve the synthesis is ethylbromide.
(10)
O
4.2) Based on the retrosynthetic analysis you devised in question 4.1, provide the necessary
reagents to transform the starting material into the desired product.
(10)
QUESTION 5:
[20]
Question type: Pericyclic Reactions and Mechanisms
5.1) Draw a full detailed mechanism of the reaction below and indicate the stereochemistry of
the product. Also, indicate the types of pericyclic reactions that are occurring.
(8)
a ret
|
HO wo
“ we
ee ao
<> a =
180°C
;
oN
“ PS ~ | aOJ N ,
|
o~
YaO
OCH;
\\
/ " OCH
HO “
aed
Page 5 of8
 |
6 Page 6 |
▲back to top |
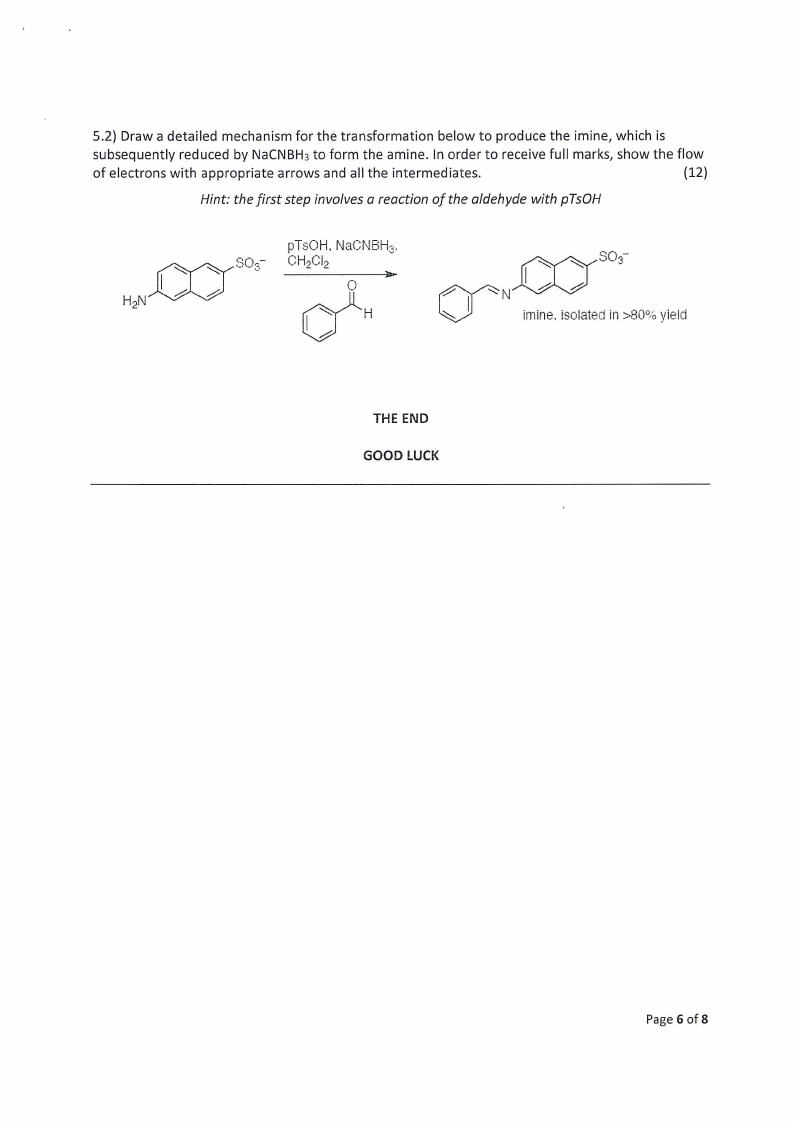
5.2) Draw a detailed mechanism for the transformation below to produce the imine, which is
subsequently reduced by NaCNBH3 to form the amine. In order to receive full marks, show the flow
of electrons with appropriate arrows and all the intermediates.
(12)
Hint: the first step involves a reaction of the aldehyde with pTsOH
HoN Cl
~
0 pCTHsCOlH., NaCNBHs,
Ta
H
0—3—
~N
imine. isolated in >80% yield
THE END
GOOD LUCK
Page 6 of 8
 |
7 Page 7 |
▲back to top |
 |
8 Page 8 |
▲back to top |