 |
AOC811S - ADVANCED ORGANIC CHEMISTRY - 1ST OPP - JUNE 2023 |
 |
1 Page 1 |
▲back to top |

n Am I BI A u n IVER s ITY
OF SCIEnCE Ano TECHnOLOGY
FACULTYOF HEALTH, NATURAL RESOURCESAND APPLIED SCIENCES
SCHOOLOF NATURALAND APPLIEDSCIENCES
DEPARTMENTOF BIOLOGY,CHEMISTRYAND PHYSICS
QUALIFICATION:BACHELOROF SCIENCEHONOURS
QUALIFICATIONCODE: 08BOSH
COURSECODE: AOC811S
LEVEL: 8
COURSENAME: ADVANCED ORGANIC CHEMISTRY
SESSION:JUNE 2023
PAPER:THEORY
DURATION: 3 HOURS
TOTAL MARKS: 100
FIRST OPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S) DR. MARIUS MUTORWA
MODERATOR: DR. RENATE HANS
INSTRUCTIONS
1. Answer ALL the questions.
2. Write clearly and neatly.
3. Number the answers clearly
4. All written work must be done in blue or black ink and sketches can
be done in pencil
5. No books, notes and other additional aids are allowed
PERMISSIBLEMATERIALS
Non-programmable Calculators
ATTACHMENTS
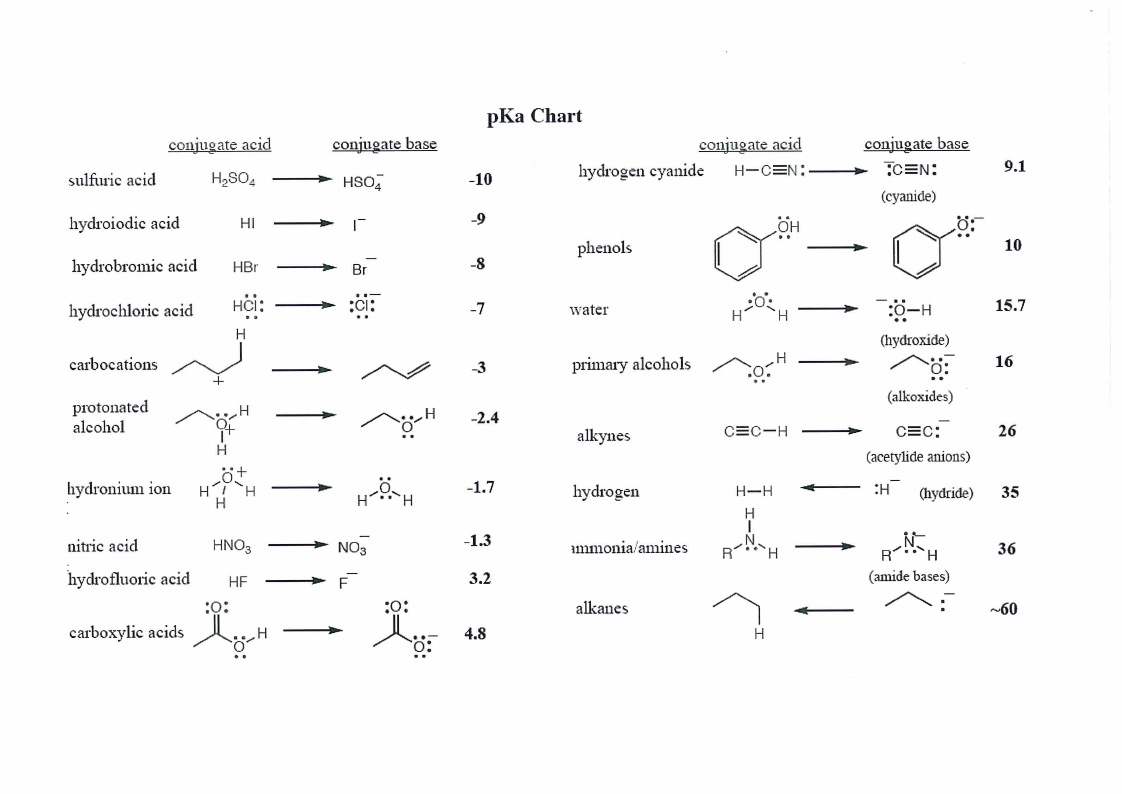
pKa Chart and Periodic Table
THIS QUESTION PAPERCONSISTSOF 7 PAGES
{Including this front page and attachments}
 |
2 Page 2 |
▲back to top |
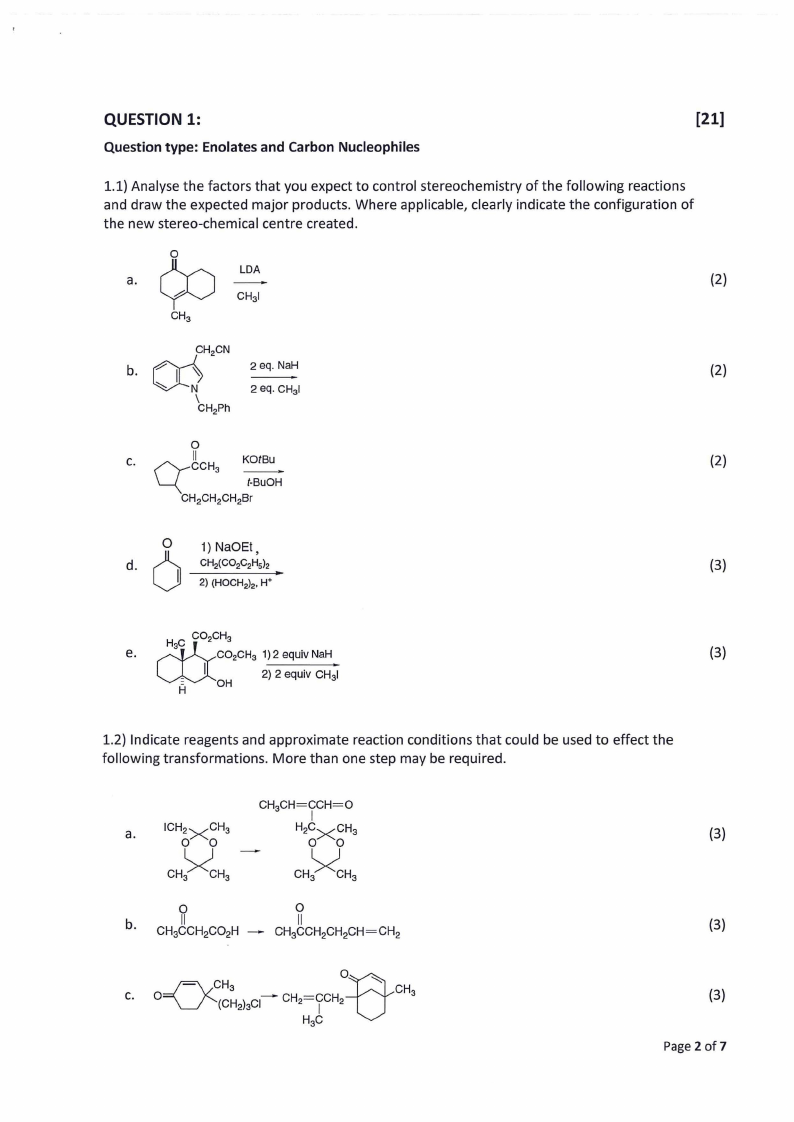
QUESTION 1:
Question type: Enolates and Carbon Nucleophiles
[21]
1.1) Analyse the factors that you expect to control stereochemistry of the following reactions
and draw the expected major products. Where applicable, clearly indicate the configuration of
the new stereo-chemical centre created.
LOA
a.
(2)
2 eq. NaH
(2)
c.
(2)
1) NaOEt,
CH2(CO2C2Hsh
(3)
2) (HOCH2)2, W
e.
(3)
1.2) Indicate reagents and approximate reaction conditions that could be used to effect the
following transformations. More than one step may be required.
a.
(3)
b.
(3)
C.
(3)
Page 2 of7
 |
3 Page 3 |
▲back to top |
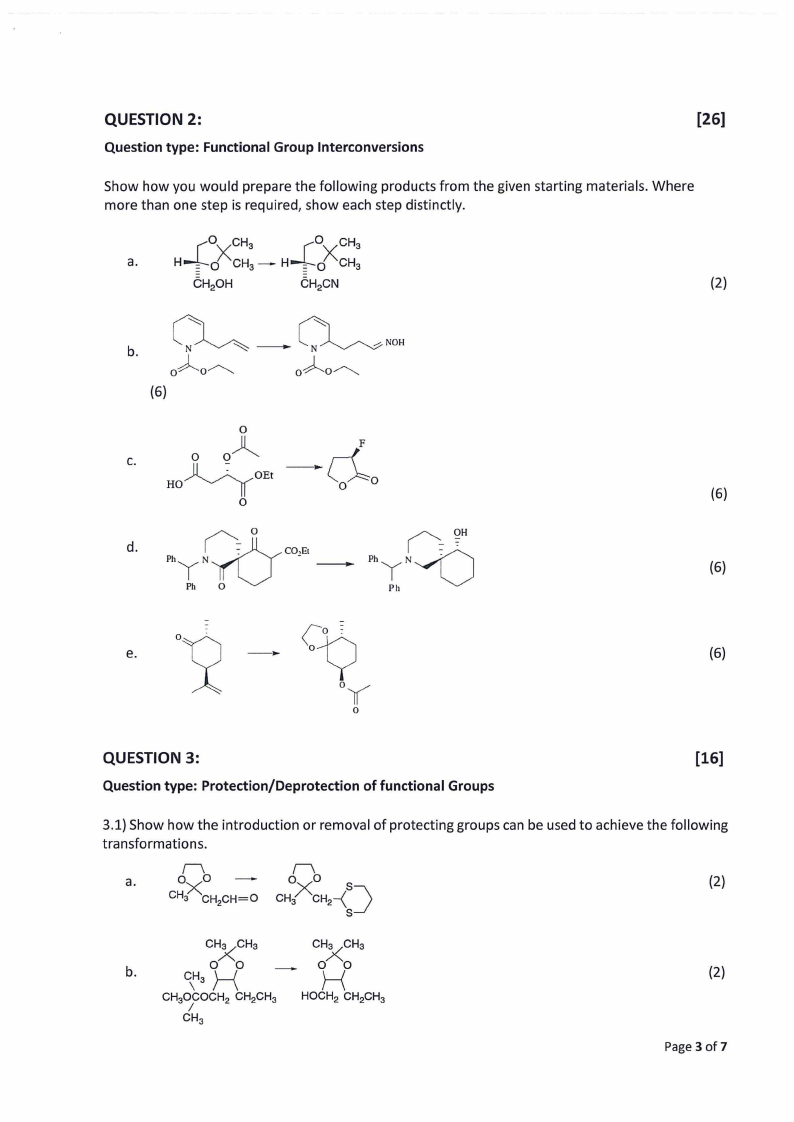
QUESTION 2:
Question type: Functional Group lnterconversions
[26]
Show how you would prepare the following products from the given starting materials. Where
more than one step is required, show each step distinctly.
a.
(2)
b.
~-~NOH
oAo~
(6)
oAo~
0
C.
0 0~
Jl If HO' '-,/
~OEt
(6)
0
U - - Ylvco,E1 d.
I/'-.__
0
PhYN'f
l_.,r\\ /'-.__
OH
PhyN-
(6)
Ph O
Ph
e.
(6)
QUESTION 3:
Question type: Protection/Deprotection of functional Groups
[16]
3.1) Show how the introduction or removal of protecting groups can be used to achieve the following
transformations.
a.
(2)
b.
(2)
Page 3 of7
 |
4 Page 4 |
▲back to top |
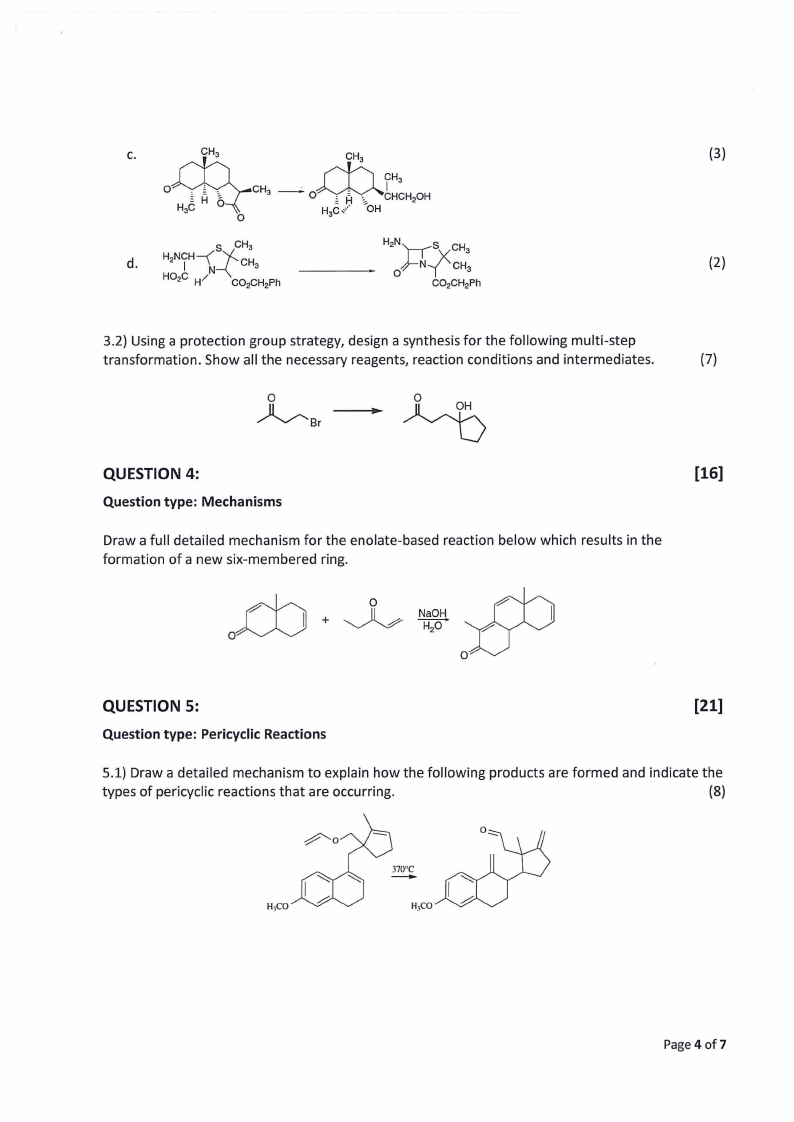
C.
(3)
d.
(2)
3.2) Using a protection group strategy, design a synthesis for the following multi-step
transformation. Show all the necessary reagents, reaction conditions and intermediates.
(7)
0
~Br
QUESTION 4:
Question type: Mechanisms
[16]
Draw a full detailed mechanism for the enolate-based reaction below which results in the
formation of a new six-membered ring.
rh
ft") 0
NaOH
H2 0
O
QUESTION 5:
Question type: Pericyclic Reactions
[21]
5.1) Draw a detailed mechanism to explain how the following products are formed and indicate the
types of pericyclic reactions that are occurring.
(8)
H,CO
H,CO
Page 4 of7
 |
5 Page 5 |
▲back to top |
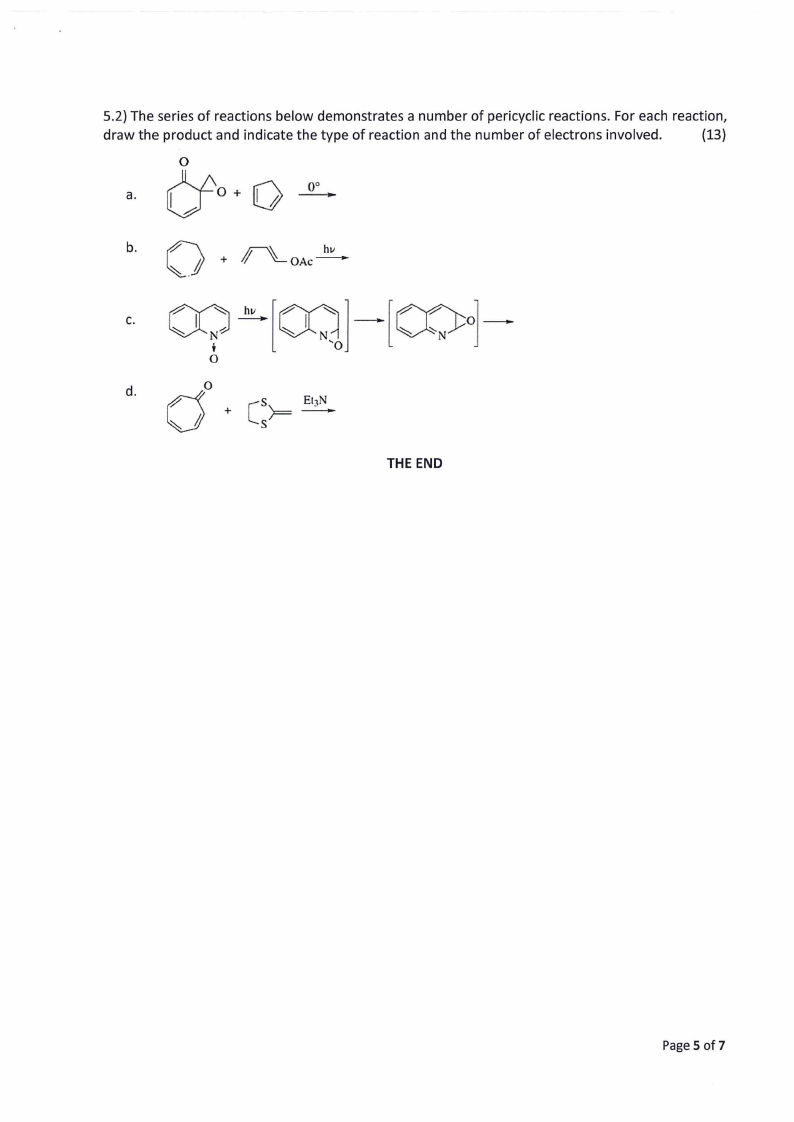
5.2) The series of reactions below demonstrates a number of pericyclic reactions. For each reaction,
draw the product and indicate the type of reaction and the number of electrons involved.
{13)
0
a. (Jo+0
b.
0
+ ~OAc~
C. 0? ~[oq]-[COo]-
0
d.
THE END
Page 5 of 7
 |
6 Page 6 |
▲back to top |
conjugate acid
sulfuric acid
H..,S..O., ,
hydroiodic acid
HI
hydrobromic acid HBr
hydrochloric acid
H.C.I:
H
carbocations
+
protonated
alcohol
/'--• OI.,+,.H
H
hydronium ion
H,,./.0.
+
'H
H
nitric acid
HNO3
hydrofluoric acid HF
:o:
)l .. carboxylic acids
0.,,H.
conjugate base
Hso;
,-
Br-
..•
:c1:
.. /'--,• .,,,H
0
..
H...0.-·.'H
• N0 3
-
F
:o:
)lo:
pKa Chart
conju·gat.eacid
conjugate base
-10
-9
hydrogen cyanide H-C=N:
:c=N:
9.1
phenols
v?,Hv~=- (cyanide)
•&
10
-8
-7
water
H,•.·..o.'H·•
.. .. - :o••-H
15.7
01ydroxide)
-3
.. primary alcohols /'-- .o.,,.H
/'--a: 16
(alkoxides)
-2.4
alkynes
c=c-H
c=c:
26
(acetylide anions}
-1.7
hydrogen
H-H
H
I
-1.3
unmonia/amines
R,,."N•' H
:H (hydride} 35
.N'-
R....-". 'H
36
3.2
alkanes
..
.. - (amide bases)
/'--,.....
~60
4.8
H
 |
7 Page 7 |
▲back to top |
h)droge1
1
H
1,0(179
lrthlum
3
Li
berytllJm
4
Be
6.911
sJdium
11
9.)122
magiesium
12
Na Mg
~2.990
po:as;lum
19
K
2L.3Q5
c£Jcium
20
Ca
~9.098
rubidium
37
40.078
stronti.Jm
38
Rb
1:5.468
caesium
55
Sr
87,62
barium
56
Cs Ba
13291
francium
87
Fr
137.33
ra:lium
B8
Ra
122'31
122nl
57-70
*
89-102
**
scan:Jium titani.Jm vmudi.Jm c1romlum mmganESe Iron
21
22
23
24
25
26
Sc Ti V Cr Mn Fe
44.956
'.lttrUIT
39
y
47.e67
zlr:onlun
40
Zr
50.9L2
51.996
54.93E
5iB45
nloblLm mclyt:denun techn3tlum rutheriun
41
42
43
44
Nb Mo Tc Ru
88.906
lute:lun
71
Lu
91.224
hafnium
72
Hf
9,.906
tantalum
73
Ta
9E.94
!Jngs1en
w74
1931
rherlun
75
Re
101.)7
osmium
76
Os
174.9i
178.49 160.95 18l.84
lawrencium rulherfordium dubnium seaborgum
103 104 105 106
·es.21
t:ohriun
107
190.23
hassium
108
Lr Rf Db Sg Bh Hs
1262)
'261)
12621
12561
12641
12691
boron
car:on
nltro,ier
5
6
7
BC N
10.811
auminlum
13
Al
12.:i1•
silicor
14
Si
14.C07
phosphoJrS
15
p
26.9B2 28)86
30.£74
cobalt
nicke
copper
zinc
gollum ge-m~nium a-senle
27
28
29
30
31
32
33
Co Ni Cu Zn Ga Ge As
5e.923
rhcdiLm
45
Rh
58.693
pJll,diLm
46
Pd
63.546
sliver
47
Ag
65.29
cadmlurr
48
Cd
69.723
Indium
49
In
72.61
tin
50
Sn
74.S22
antlmon-(
51
Sb
1C2.S1
iridium
77
Ir
106.42
platnu11
78
Pt
10787
god
79
Au
112.41
mercury
80
Hg
114.82
1h~lllum
81
Tl
118.7'
leJd
82
Pb
121,76
blsmu1h
83
Bi
1£2.,2
195.08
19697
200.59
meiherium ununnilium unununium Jnunbum
109 110 111 112
20U8
207.2
JnLnquadurr
114
208.98
Mt Uun UuuUub Uuq
1268/
12711
(2721
12771
12891
0>ygen
8
0
15.999
sulfur
16
s
32.065
sel9n1Jm
34
Se
78.~
tellurium
52
Te
127.60
polJnlum
84
Po
12091
fluorlre
9
F
1899S
:hlorine
17
Cl
'35453
mrnlne
35
Br
79904
Iodine
53
I
123.9:)
Jsb11ne
85
At
12101
1eli.Jm
2
He
4.0021:
nron
10
Ne
20.18C
arg:rn
18
Ar
39.94E
trypton
36
Kr
83.80
~eron
54
Xe
131.29
racl:Jn
86
Rn
12221
lanthJnum
* Lanthanideseries
57
La
** Actinideseries
138.9'
~c1inlum
89
Ac
12271
cerium prose,d)mui rr neodymium prometh!Jm samariun
58
59
60
61
62
Ce Pr Nd Pm Sm
14012
thori.Jm
90
Th
1L0.91 144.24
prot3Ctil1Ull Jranrum
91
92
Pa u
11451 150.36
neptLnlLm plutorlun
93
94
Np Pu
'.;3204
231.04
233.03
12371
124-11
eurJpiJm gadollniJm terbUIT dyspnJ;lu11 holmium
63
64
65
66
67
Eu Gd Tb Dy Ho
151.96
americium
95
157 .25
curlun
96
'59.93
162.30 1E4.£3
berkelium cali'ornlum elnsreinlum
97
98
99
Am Cm Bk Cf Es
124~
12471
12471
12511
12521
:rtlum
68
Er
thulium ytterburr
69
70
Tm Yb
167.26
16893
173.04
fermium mendelevum nobeliurr
100 101 102
Fm Md No
12571
12!':81
12591
Page 7 of 7





