 |
ICA511S - INTRODUCTION TO CHEMISTRY - 1st Opp - JUNE 2022 |
 |
1 Page 1 |
▲back to top |
nAm I BIA UnlVERSITY
OF SCIEnCE AnD TECHnOLOGY
FACULTY OF HEALTH, APPLIED SCIENCESAND NATURAL RESOURCES
Department of Agriculture and Natural Resources Sciences
QUALIFICATIONS: BACHELOR OF AGRICULTURE
BACHELOR OF HORTICULTURE
QUALIFICATIONS CODE:
07BAGA & 07BHOR
LEVEL: NQF LEVEL 5
COURSE CODE: ICASllS
COURSE NAME: INTRODUCTION TO CHEMISTRY
DATE: JUNE 2022
SESSION: JUNE
DURATION: 3 HOURS
MARKS: 120
EXAMINER:
MODERATOR:
FIRST OPPORTUNITY EXAMINATION QUESTION PAPER
MS. PAULINA NDINELAGO NAUPU
MRS. LUCIA TUYENI-KELAO KAFIDI
INSTRUCTIONS
1. Answer all questions
2. Type clearly and neatly
3. Number the answers clearly
4. Report all your answers to the correct significant figures
PERMISSIBLE MATERIALS
1. Scientific calculator
ATTACHMENT:
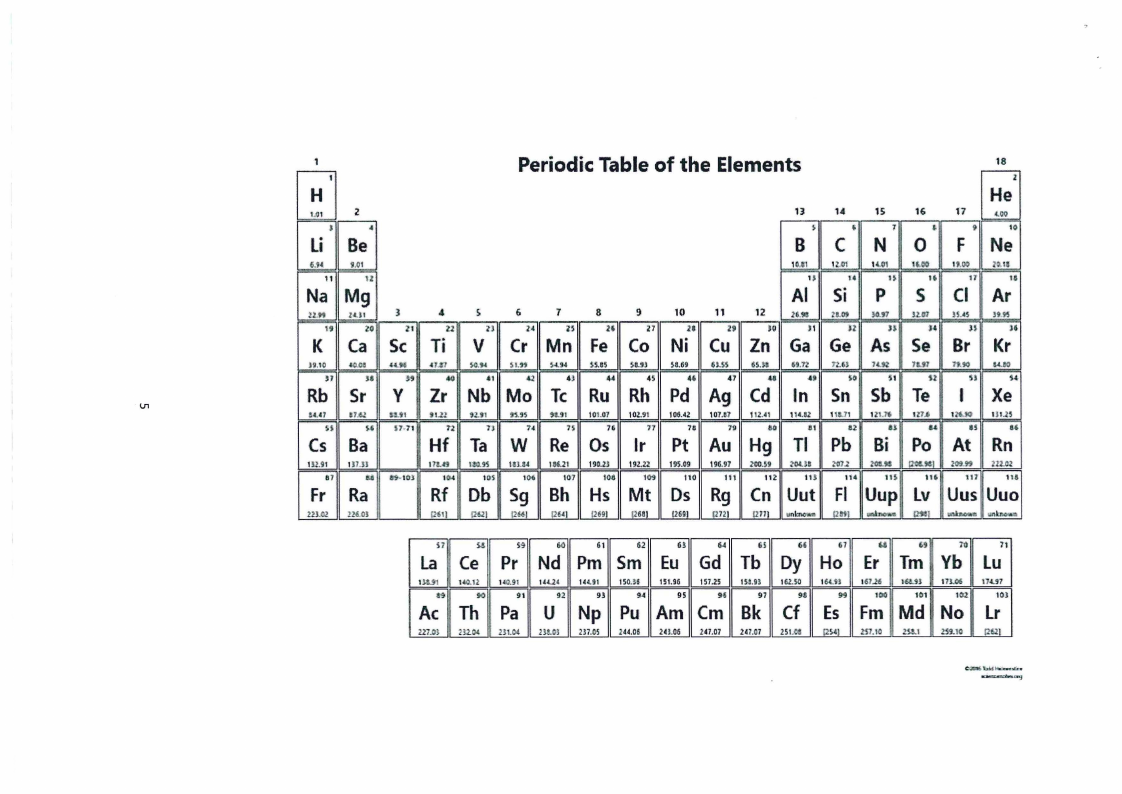
1. Periodic Table
THIS QUESTION PAPERCONSISTSOF 4 PAGES(Excluding this front page)
0
 |
2 Page 2 |
▲back to top |

QUESTION 1
Define the following terms:
[14]
i. Atoms
ii. Element
iii. Molecules
iv. Precision
V. Accuracy
vi. Conversion factor
vii) Periodic table
QUESTION 2
Work on the following questions:
[13]
a) The distance between NUSTand UNAM 48km. What is the distance between NUST
and UNAM in centimeters
{3}
b) A rock has a mass of 20.5 g and a volume of 15.05 cm3. What is its density?
{5}
c) A rock has a density of 18.3 g/cm 3• If you have a rock bar with a volume of 43.9 cm3,
what is its mass?
{5}
QUESTION 3
State the four Dalton's theory and give an example/illustration of each theory. [8]
1
 |
3 Page 3 |
▲back to top |

QUESTION 4
a) Magnesium has three isotopes with mass numbers 24, 25, and 26.
[14]
i. Write the complete chemical symbol (superscript and subscript) for each
{3}
ii. How many neutrons are in an atom of each isotope?
{3}
b) Draw the ionic bond between magnesium and bromide. Clearly show how electron
are transferred/shared/lost and the resulting ions
{8}
QUESTION 5
a) Provide the empirical formula of the following compounds.
[8]
iii) CsH100s
bj For each of the following identify it as either ionic or molecular compound. For
ionic, indicate the charges of each element.
[12]
ii) MgCb
iii) CO2
v)
Sr(OH)z
2
 |
4 Page 4 |
▲back to top |

QUESTION 6
a) What is the mass of 0.30 moles Mg(N03}z
[5]
b) Balance the following equations
[11]
ii) Mg+ N2
c) Calculate the formula weight (FW) of the following substances Potassium bromide
[9]
iii) KMn04
3
 |
5 Page 5 |
▲back to top |

QUESTION 7
Calculate the percentage composition of carbon in the following substances.
[10]
{5}
{5}
QUESTION 8
2 moles of propane reacts with 8 moles of oxygen gas in a combustion reaction in the
following equation: lC3Hs + 502----+3C02 + 4H20.
[16]
a) What is the limiting reactant
{6}
b) How many moles of carbon dioxide are formed
{5}
c) How much of the excess reactant is left over?
{5}
Total Marks:
120
4
 |
6 Page 6 |
▲back to top |
1
Periodic Table of the Elements
18
1IU~BIe~'• 1.
t.A)I
H
1Z
13
,, 1.5
16
17
5
7
.. 9
10
B
,o..,
IJ
CN
,. u.o. f
14.01
1$
0 F i Ne
I
IUO
IMO 1 ll>.11,
.. 17
If
Na Mg
Al Si p s Cl Ar
n.tt lUt
it
io
3
21
' 2l
s
l)
6
i"
7
lS
8
H
9
10
11
l'i
28
ii)
,~ 12
2UG
,;
ta.Of
u
J0..'7
J)
,. J.to7
ls.-1~ !t.'1
t~
.u'
V,
K
!HO
Ca Sc
~, «.M
,, • ., ., ... .. H
Rb
G ..-.....!!!L
S:S
rV
.
s,
s·u,
n.11
Cs Ba
G E] ~, [:' nu, UUl
G8 "·'!~2 '~~:88--'" 0 ...E., . H,O>
Ti
41.$1
Z,,ur
71
Hf
ll&...,
104
Rf
j2_!11_
V
SC.OM
Nb
,u,
1j
Ta
1!11~
-·---
Cr
si.;t
Mo
tUS
4
Mn
s.a.,~
Tc
,u,
75
Re
lSd.21
.
Fe
SU$
.«
Ru
101.01
76
Os
190.21
-
··-
..
Co
SU)
4S
Rh
102.91
77
Ir
1!l2.22
- ··- ---
Ni Cu Zn Ga Ge As Se
~$.69 nss
«
,uo
, '9.12
1UJ
14.~
JO
St
lU7
n
Pd Ag Cd Sn Sb Te 106.42
107.e?
11u,
In
114.IU
,,u,
Ul,7'
,v ..
711
79
ao
!1
u
u
Pt Au Hg Tl
Bi Po
195.09 196.97 200.~9 t{JUS
2:0a.tt
It 1
'112
HS
Rg Cn Uut
11S
Uup lv
1_2__!21 1,m1 unl._
I ""'-
!lt-'1
Br
'"'°$l
I
I~
I!
At
20il.99
Kr
S-00
µ
Xe
Ul.25
.
Uus 1 Uuo
-
' """'-"
S7
61
1>2
63
6§
'9
10
n
la
u
"B 8 GJEG ll"JI
'~-~
Pm Sm Eu
UUI
tS0.!8 151.96
Tb
UU!
~?,
Tm Yb Lu
JQ;.9j
17U6
l7.U7
~EJG.. ~8 "' Ac" Th•Pa
U
9~
95
96
97
9l!
Np Pu Am Cm Bk Cf
11)2
1(1)
Fm Md No Lr
U7.J)J 23204. Bl.l>t
2l8.0l
237.05 244.06 243.06 H1.01 20.07
251.~8
S7.IO
is&.1
259..10
[2~21
~~~.lb.•
-"'9





