 |
ACS701S - APPLIED COLLOID AND SURFACE CHEMISTRY - 1ST OPP - JUNE 2022 |
 |
1 Page 1 |
▲back to top |

A\\)
NAMIBIA UNIVERSITY
OF SCIENCE AND TECHNOLOGY
FACULTY OF HEALTH, APPLIED SCIENCES AND NATURAL RESOURCES
DEPARTMENT OF NATURAL AND APPLIED SCIENCES
QUALIFICATION: BACHELOR OF SCIENCE
QUALIFICATION CODE: 07BOSC
LEVEL: 7
COURSE CODE: ACS7015S
COURSE NAME: APPLIED COLLOID AND SURFACE
CHEMISTRY
SESSION: JUNE 2022
DURATION: 3 HOURS
PAPER: THEORY
MARKS: 100
FIRST OPPORTUNITY EXAMINATION QUESTION PAPER
EXAMINER(S) | Prof Habauka M. Kwaambwa
MODERATOR: Prof Edet F. Archibong
INSTRUCTIONS
Answer ALL the FIVE questions
Write clearly and neatly
Number the answers clearly
All written work must be done in bule or black ink
No books, notes and other additional aids are allowed
Mark all answers clearly with their respective question numbers
PERMISSIBLE MATERIALS
Non-programmable Calculators
ATTACHMENT
List of Useful Constants
THIS QUESTION PAPER CONSISTS OF 6 PAGES (Including this front page and List of Useful
Constants)
 |
2 Page 2 |
▲back to top |

QUESTION 1
[23]
(a) Colloids can be described in terms size, dispersed phase/dispersion medium, and
lyophilic or lyophobic colloids. Write briefly about this statement.
(5)
(b) Surfactants are classified according to the type of the hydrophilic group. In many
respects, the aqueous behaviour of dodecybetaine is closer to octyl poly(oxyethylene)
glycol (Triton X-100) than to hexadecyltrimethylammonium bromide. Explain or
discuss this statement.
(4)
(c) Water treatment Moringa seed proteins are cationic. Arrange in increasing order of
interaction of the following surfactants with Moringa seed proteins and explain briefly
your answer:
Cetylpyridinium bromide; Polyoxyethylene alkyl ether, and; Sodium dodecyl sulphate.
Explain your answer.
(4)
(d) There is a variety of physical properties that can be used to determine the critical
micelle concentration (CMC) of a surfactant such as sodium dodecyl sulphate (SDS).
On the same diagram, show the variation of the following physical quantities with SDS
concentration, showing clearly the position of the CMC:
(4)
(i)
Osmotic pressure
(ii)
Turbidity
(iii)
Surface tension
(e) Explain briefly the observed behaviours in (d) above.
(6)
QUESTION 2
[27]
(a) Define the terms solubilisation, Krafft temperature, Tx, and cloud point as used in
colloid chemistry.
(6)
(b) The cloud point for TX-100 was studied as function temperature. What would you
observe if the same experiment was done using sodium dodecyl sulphate (SDS)? (2)
(c) Using well-labelled schematic diagrams illustrate how (i) solubilisation varies with
surfactant concentration, and; (ii) how solubility of surfactants with temperature
indicating clearly the position Tx and critical micelle concentration.
(7)
(d) State whether the critical micelle concentration (CMC) would increase, decrease or
not change after the following changes:
(5)
(i)
Changing the surfactant from CH3(CH2)9(OCH2CH2)sOH to
CH3(CH2)7(OCH2CH2)s50H
(ii)
Increasing the temperature
(iii)
Addition of electrolyte to an ionic surfactant
(iv)
Presence of impurity when CMC is determined by surface tension
(v)
Branching of the hydrophobic part of the surfactant
2
 |
3 Page 3 |
▲back to top |

(e) A Moringa shower gel formula has the following components:
Identification | Component
A
Water
SDS 30%
Coconut diethanolamide
Alkyl amido propyl betaine 30%
Cocoamine oxide
NaCl
Perfume, colour, preservative
Lactic acid
Moringa seed oil
Match each of the following functions to corresponding letter (A-J) of the components
in the table above:
(7)
(i)
Increases thickness by causing the surfactant to restructure into the high
viscosity cylindrical micelle structures
(ii)
Anionic surfactant
(iii) Thought to be a component of the skin and used to the pH compatible with
that of the skin, i.e. adjust pH to 6.5
(iv) Antiaging and antifungal component
(v)
Amphoteric surfactant to generate foam or cold water detergent
(vi)
Nonionic surfactant that imparts excellent viscosity enhancing and foam
stabilisation in anionic based systems
(vii) Foam booster surfactant
QUESTION 3
[12]
(a) Outline any three main assumptions involved in the derivation of the BET adsorption
isotherm equation for molecules at the gas/liquid interface.
(3)
(b) The linear
_ Po
BET
leL qu%ati(oCn-Dis pof
the
form:
V(po- P) Vane Van Po
(i)
State what each of the quantities in this equation represents.
(4)
(ii)
A graphical plot of Yoon against P data for the adsorption of nitrogen
Po ~ p
Py
gas on 1g of sample of alumina at 77 K gave a slope of 2.88 x 10% cm? (s.t.p.)
and an intercept of 9.87 x 10 cm’ (s.t.p.). Calculate the specific surface area
(m*g*) for the alumina sample, taking the molecular area of nitrogen as
16.2 x 10°79 m2.
(5)
 |
4 Page 4 |
▲back to top |

QUESTION 4
[20]
(a) Compare and contrast the following terms as used in colloid stability:
(6)
(i)
Sedimentation and Creaming
(ii)
Depletion flocculation and Bridging flocculation
(b) Explain how the following factors would affect the stability of colloidal dispersions.
(6)
(i)
Brownian motion
(ii)
Increase in particle size of colloidal particles
(iii)
Decrease in medium viscosity
(c) Define the terms point of zero charge and potential determining ions. As a Colloid
Scientist, use/apply these concepts to explain why Agl particles are negatively
charged and how you can manipulate them so that you have a dispersion with zero
charged Agl particles and another with positively charged Agl particles.
(6)
(d) Apart from the above mechanism in (c), isomorphous substitution is another
mechanism particles acquire charge. Deduce the resulting charge of clay particles if
metal X (valency = 4*) replaces metal M (valency = 3*)?
(2)
QUESTION 5
[18]
(a) One form of the van Waals interaction potential between two particles is given by:
Aa
Va(h) = 12h’
(i)
State any two conditions under which this equation is valid.
(2)
(ii)
Briefly state the effect on Va if the particles are immersed in medium instead
particles instead of particles in a vacuo?
(1)
(iii) What effect on Va is observed if the Hamaker constant of the medium
approaches that of the particles?
(1)
(b) Using combining relations based on the Hamaker constants of pure materials (Aj),
calculate the composite Hamaker constants for the following interacting systems:
(i) Polystyrene in water; (ii) SiOz in water, and; (iii) Polyestyrene-Water-SiO2
(6)
Comment on the results with respect colloid stability of the systems.
(3)
Given:
Material
Polystyrene
SiOz
Water
Aix 10°J
7.2
0.8
4.1
 |
5 Page 5 |
▲back to top |

(c) On the same well-labelled diagram, show schematically the variation of the van der
Waals attraction potential (Va), electrostatic potential (Vr) and total pair potential (Vr
= Va + Vr) with the interparticle separation, h, for a marginally stable dispersion of
nanoparticles indicating clearly the positions, if any, of primary minimum, primary
maximum, secondary minimum and Born repulsion potential (Vs).
(5)
END OF EXAM QUESTIONS
 |
6 Page 6 |
▲back to top |
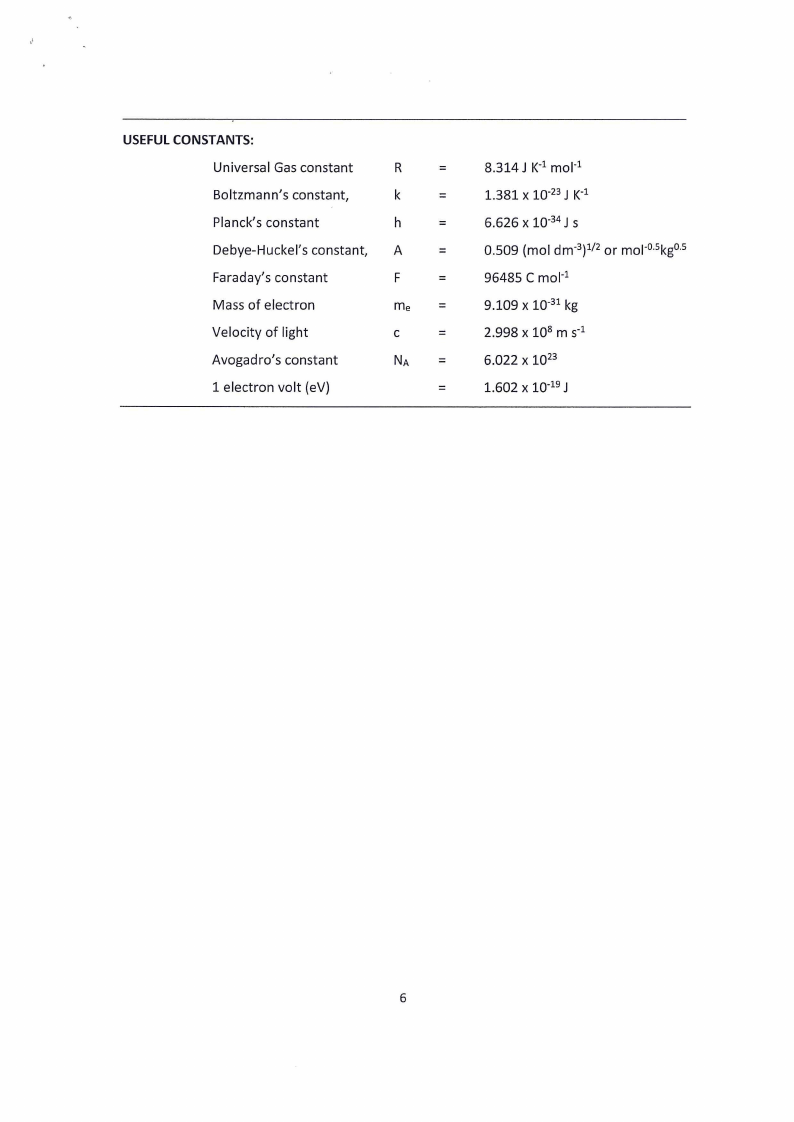
USEFUL CONSTANTS:
Universal Gas constant
Boltzmann’s constant,
Planck’s constant
Debye-Huckel’s constant,
Faraday’s constant
Mass of electron
Velocity of light
Avogadro’s constant
1 electron volt (eV)
8.314J K? molt
1.381 x 10°73J K?
6.626 x 1034J s
0.509 (mol dm3)*/2 or mol®5kg®5
96485 C mol?
9.109 x 10°31 kg
2.998 x 108 ms?
6.022 x 1073
1.602 x 10°19J





