 |
CLC611S - CLINICAL CHEMISTRY 2A - 2ND OPP - JULY 2022 |
 |
1 Page 1 |
▲back to top |

nAmlBIA unlVERSITY
OF SCIEnCE Ano TECHnOLOGY
FACULTYOF HEALTH,APPLIEDSCIENCESAND NATURALRESOURCES
DEPARTMENT OF HEALTHSCIENCES
QUALIFICATION: BACHELOR OF MEDICAL LABORATORY SCIENCES
QUALIFICATION CODE: 08BMLS
LEVEL: 6
COURSECODE: CLC611S
COURSENAME: CLINICAL CHEMISTRY 2A
SESSION:
JULY 2022
PAPER:
THEORY
DURATION: 3 HOURS
MARKS:
100
SUPPLEMENTARY/SECOND OPPORTUNITYEXAMINATION QUESTION PAPER
EXAMINER{S)
Dr Elzabe van der Coif
MODERATOR:
Dr Maurice Nyambuya
INSTRUCTIONS
1. Answer ALL the questions.
2. Write clearly and neatly.
3. Number the answers clearly.
PERMISSIBLEMATERIALS
1. Calculator
2. Attachment Levey-Jennings chart
THIS QUESTION PAPERCONSISTSOF 8 PAGES(Including this front page)
 |
2 Page 2 |
▲back to top |

QUESTION 1
[10)
The following question relates to specimen collection and specimen
handling. Fill in the missing words from the list provided - only write the
question number and the missing word/s.
unlabelled
infectious
identity
name of the patient
jaundice
patient preparation
confident
K+
well-organized
stressed
anaemia
LD
1.1 A phlebotomist should be ____
and ____
_
1.2 He/she should make sure of the
of the patient before
sample collection.
1.3 The phlebotomist should write the _____
on the tube AFTER
collection.
1.4 A laboratory should never receive a sample which is ___ _
1.5 Treat all specimens as if ____
_
1.6 A haemolysed blood sample is unfit for analysis since the ___
and
____
would be raised.
1.7 Heel pricks are performed on newborn babies to test for blood bilirubin
levels in the case of ____
_
1.8 The laboratory is responsible to ensure correct _____
before
sample collection, eg. fasting.
QUESTION 2
[12)
On Tuesdays the Clinical Chemistry Laboratory receives blood samples
from patients who visit the Out-Patients Department (OPD) at the
hospital. They are diabetics who come to have their blood glucose levels
monitored.
9:00: You receive 20 blood samples for blood glucose testing. Each
sample has a barcode and the patient's name written on the tube
9:15: You centrifuge the samples and load them into the automated
analyser
10:00: You transfer the results from the analyzer to the LIS using the
computerized system
10:15: Your supervisor signs off the results on the LIS
11:00: The doctor phones from OPD saying that he received a blood
glucose result of 2 mmol/L for patient X. It means the patient should be
hospitalized and treated immediately. He questions the result since the
2
 |
3 Page 3 |
▲back to top |

patient was fine with the previous visit.
You will have to investigate and prove to the doctor that your result is
correct.
Answer the following questions regarding this case scenario.
2.1 Identify possible errors in the pre-analytical phase of laboratory testing. 2
2.2 Describe the patient preparation for a fasting blood glucose test.
1
2.3 Identify possible errors in the analytical phase of laboratory testing.
Outline what you would check, and possible causes of random and
systematic errors.
5
2.4 What information is stored in the analyzer which you could use as
evidence that your result is correct?
2
2.5 Identify possible errors in the post-analytical phase of laboratory testing. 2
QUESTION 3
[3]
Identify three (3) safety hazards in a clinical chemistry laboratory.
QUESTION 4
[12]
When a patient suffers from liver cancer the alpha fetoprotein (AFP)
level in the blood is raised. You tested a serum sample from a patient in
the automated analyzer and the AFP level was out of the linear range of
the instrument. A dilution needed to be made. You made a 1/10 dilution
of the serum sample and re-run the test. The result was still out of the
linear range of the instrument. Answer the following questions: 12
4.1 Which solution is commonly used in clinical chemistry as a diluent to
make a dilution of serum.
1
4.2 Explain the name given to the solution in 4.1. Referring to what?
1
4.3 The percent concentration of physiological saline is 0.9%. Explain how
you would prepare 100 ml of physiological saline.
3
4.4 Mention which grade of water you would use for physiological saline and
how it is prepared.
2
4.5 Describe how you would make a 1/100 dilution of the serum. You have
2 ml serum available, and the analyzer requires 200 ul of diluted sample
for analysis. Taking into consideration that pipetting of less than 10 ul
3
 |
4 Page 4 |
▲back to top |
serum is not very accurate, you decide to use 10 ul of serum. Calculate
the total volume and the volume of diluent needed.
3
4.6 The second dilution is re-run in the analyzer and the result is 10.58 IU/ml.
Which result should be reported? Remember the unit of measurement.
2
QUESTION 5
[8]
Match the definitions in column 1 with the correct terms in column 2.
Each term should be used once. {One mark each.)
Column 1
Column 2
5.1 95% confidence limits
A.Mode
5.2 Substance treated exactly as a patient
sample; used to detect analytical errors
5.3 Represents the smallest concentration
that a test can measure
5.4 Comparison of a patient result with a
previous result
5.5 Proportion of persons with a disease who
test positive
5.6 The average value in a set
B.Mean
C.Diagnostic
sensitivity
D.Diagnostic
specificity
E.Analytical
sensitivity
F.25D
5. 7 Proportion of persons without disease who
test negative
5.8 Most common value in a set
G.Control
H.Delta check
QUESTION 6
[15)
The question relates to quality assurance. Consider the quality control
done on the total protein test over a period of 10 days. The following
values were obtained with the low protein control sample {Level 1).
Day
Low control total protein {g/I)
1
40
2
42
3
60
4
 |
5 Page 5 |
▲back to top |

4
43
5
32
6
34
7
31
8
33
9
38
10
42
The manufacturer of the control material provided the following
information for the low control:
Mean: 40 g/1
One standard deviation: 5 g/1
Answer the following questions.
6.1 Indicate the mean value which you will insert in the Levey-Jennings chart 1
6.2 Calculate the value of one standard deviation above the mean.
1
6.3 Calculate the value of two standard deviations above the mean.
1
6.4 Calculate the value of one standard deviation below the mean.
1
6.5 Calculate the value of two standard deviations below the mean.
1
6.6 Use the information in your calculations above and plot the low control
values for total protein over the ten days as indicated in the table above.
Detach the provided graph paper and attach it to your answer sheet.
5
6.7 Identify any day where there was an out-of-control incident and indicate
which Westgard rule was violated.
2
6.8 Was the error random or systematic?
1
6.9 Identify one more violation of the Westgard rules.
1
6.10 Describe possible corrective action for any of the errors above.
1
5
 |
6 Page 6 |
▲back to top |
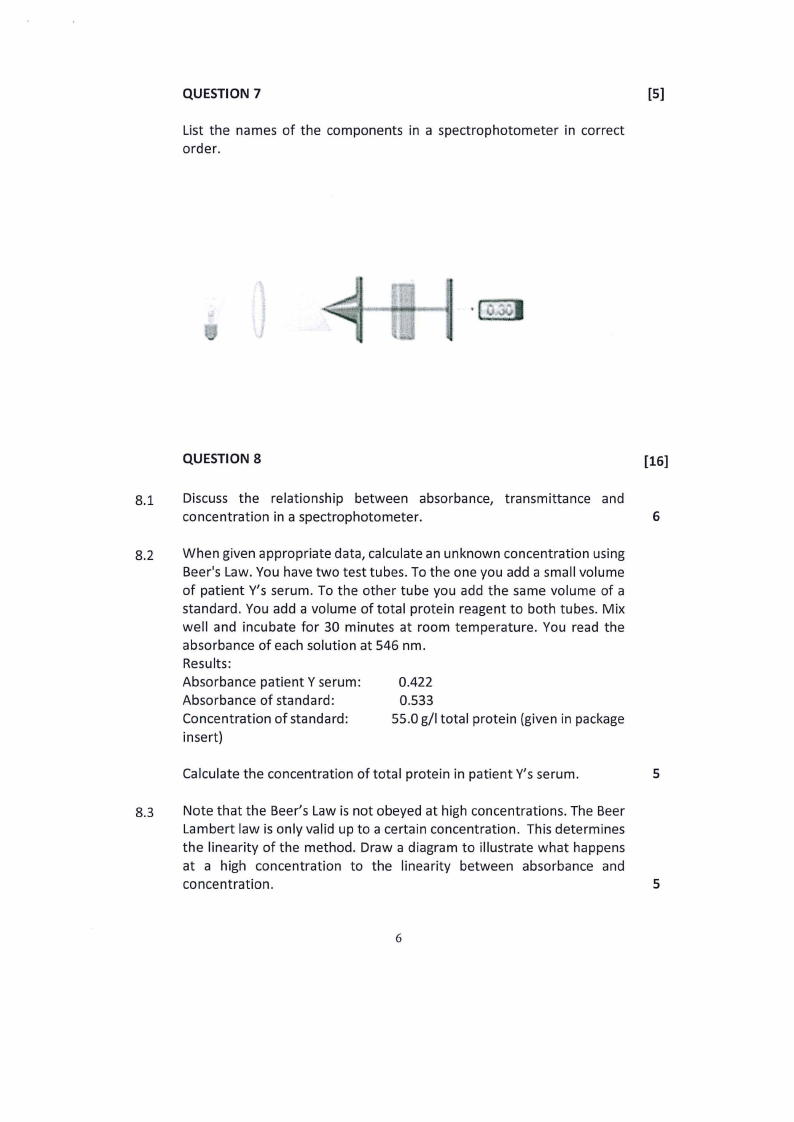
QUESTION 7
[S]
List the names of the components in a spectrophotometer in correct
order.
QUESTION 8
[16]
8.1 Discuss the relationship between absorbance, transmittance and
concentration in a spectrophotometer.
6
8.2 When given appropriate data, calculate an unknown concentration using
Beer's Law. You have two test tubes. To the one you add a small volume
of patient Y's serum. To the other tube you add the same volume of a
standard. You add a volume of total protein reagent to both tubes. Mix
well and incubate for 30 minutes at room temperature. You read the
absorbance of each solution at 546 nm.
Results:
Absorbance patient Y serum:
0.422
Absorbance of standard:
0.533
Concentration of standard:
55.0 g/1total protein (given in package
insert)
Calculate the concentration of total protein in patient Y's serum.
5
8.3 Note that the Beer's Law is not obeyed at high concentrations. The Beer
Lambert law is only valid up to a certain concentration. This determines
the linearity of the method. Draw a diagram to illustrate what happens
at a high concentration to the linearity between absorbance and
concentration.
5
6
 |
7 Page 7 |
▲back to top |
QUESTION 9
9.1
Explain the basic principle of chemiluminescence
5
9.2
State a common laboratory application for chemiluminescence. Used to 2
detect which analytes?
9.3
Discuss the advantages of using chemiluminescent techniques over
other methods.
4
QUESTION 10
[8]
Match each description in column 1 with the correct term in column 2.
Column 1 Description
10.1.Commonly used
label
chemiluminescent
Column 2
A.Blood gases
10.2. analytes which are measured with gas
sensing ion selective electrodes
10.3. analytes which are measured with ion
selective electrodes
10.4. a protein that combines with a specific
antigen
B. Antibody
C. Affinity
D. Luminol
10.5. a substance capable of inducing the
formation of an antibody; the substance
which binds to an antibody in an
immunoassay
10.6. strength of the bond between an
antigen and an antibody
10.7. attached to either an antigen or
antibody, used to differentiate the patient
analyte from reagent
10.8.an advantage of automation in the
clinical chemistry laboratory
E. Improvement of TAT
F. Labels
G. Serum electrolytes
H. Antigen
7
 |
8 Page 8 |
▲back to top |
' '· f
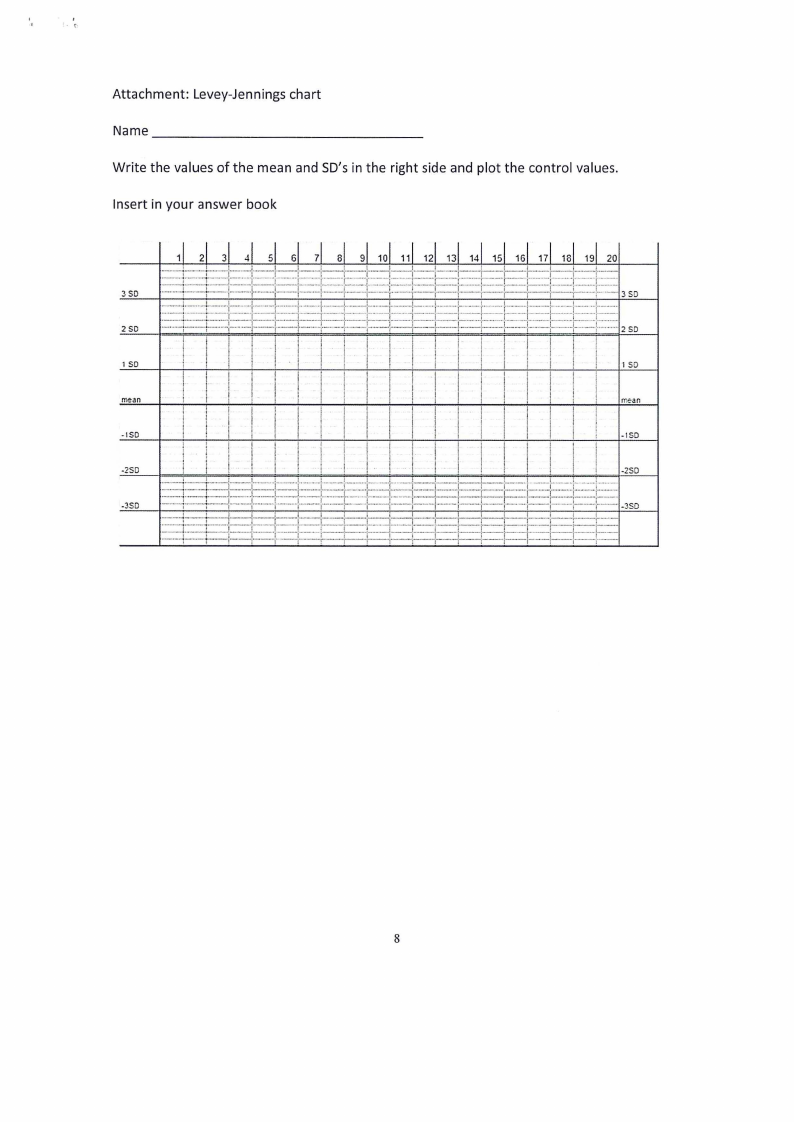
Attachment: Levey-Jennings chart
Name _________________
_
Write the values of the mean and SD's in the right side and plot the control values.
Insert in your answer book
3 SD
2 SD
l
I
1 SD
mean
-lSD
-2SD
1---------;- -3SD
1----;---t--+
1---+--+: --, 1'- I :
1--...-------i----i'---1-·- ··--:------i--·-I
1 SD
mean
-lSD
-2SD
: I l ' I -·--:,---.---.--,
:, -·,l----·l,::._--__-_-__..-._,_'.1:;1_,,-_·---,!_-_,--:L-_-_-_--·--
8





